IL-36 Cytokines: An Emerging Target for Inflammatory Diseases
IL-36, A Member of IL-1 Superfamily, Emerging Target for Treating Inflammatory Diseases
Introduction to IL-36 Family
Twenty years ago, Interleukin 36 (IL-36) was discovered as a member of the IL-1 superfamily. Its gene is located on chromosome 2 in an IL gene cluster, including IL1A - IL1B - IL37 - IL36G - IL36A - IL36B - IL36RN - IL38 - IL1RN, with IL-36 encoding genes transcribed far away from the centromere.
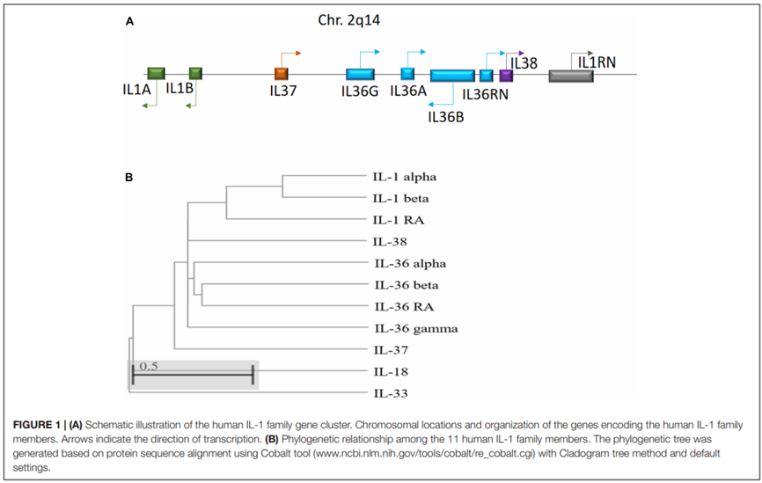
Figure 1. Schematic diagram of the human IL-1 family gene cluster and the phylogenetic relationship among the 11 IL-1 family members
There are four known subtypes of IL-36, which have been renamed multiple times and were formerly known as IL-1F6, IL-1F8, IL-1F9, and IL-1F5. Ten years ago, due to their functional definition, they were named IL-36α, IL-36β, IL-36γ, and IL-36 receptor antagonist (IL-36 Ra). The IL-36 receptor (IL-36R), also known as IL-1Rrp2 or IL1RL2, was finally confirmed as IL-1R6, which binds to all members of the IL-36 family: IL-36α, IL-36β, IL-36γ, and IL-36 Ra. Additionally, IL-38 can also bind to this receptor. Both IL-36 and IL-36R (IL-1R6) are expressed in various tissues, but IL-36R expression is highest in the skin, mammary gland, and mucosal epithelial cell lines.


Figure 2. IL-36 Family Cytokines
Molecular Structure of IL-36
The structure of IL-36 is similar to other typical IL-1F members, all having a hydrophobic core and a beta-trefoil structure, lacking a signal peptide (a classic feature of IL-1F members) and a caspase 1 cleavage site. N-terminally truncated IL-36α, IL-36β, and IL-36γ are 1000 to 10,000 times more active than the non-truncated cytokines, and the antagonistic activity of IL-36Ra depends on the removal of its N-terminal glutamic acid. IL-36Ra, starting from Val-2, has full antagonist activity, inhibiting not only IL-36γ but also IL-36α and IL-36β. Its Val-2 is the 9th amino acid of a common A-X-Asp conserved repeat at the N-terminus of all IL-1F members.

IL-36 Signaling Pathway
The IL-36R pathway is significantly similar to the IL-1R pathway, with the IL-36R composed of an extracellular ligand-binding domain, transmembrane helix, and intracellular Toll/IL-1 receptor domain (Toll/IL-1 receptor domain, TIR domain). The extracellular part is composed of three Ig-like domains, and specific residues required for ligand binding have been identified. IL-36α, IL-36β, and IL-36γ first bind to IL-36R, then form a signal-transducing complex with IL-1RAcP, recruit myeloid differentiation factor 88 (MyD88) and interleukin-1 receptor-associated kinases like 1 and 2 (IRAKs), and activate the mitogen-activated protein kinases (MAPK) and nuclear factor kappa B (NF-κB) pathways. Activation of MAPK leads to transcription of inflammation genes regulated by AP-1 (activator protein-1), while phosphorylation of Ikβ α (a negative regulator of NF-κB) by Ikβ kinase leads to the translocation of NF-κB to the nucleus, aiding in the transcription of key inflammation genes, thus generating numerous inflammatory mediators and mediating inflammatory responses, playing an important role in adaptive immunity. The binding of IL-36 Ra to IL-1R6 does not lead to the recruitment of IL-1RAcP. Therefore, the pro-inflammatory cascade reaction is not initiated, realizing the anti-inflammatory characteristics of IL-36 Ra. IL-38 also binds to IL1R6 and has anti-inflammatory effects similar to IL-36 Ra.

Figure 4. IL-36/IL-36R Signaling Pathway
IL-36 is mainly distributed in skin, lungs, joints, intestines, kidneys, and the brain, and can be produced by various cells such as monocytes/macrophages, T lymphocytes, B lymphocytes, keratinocytes, M2 cells, Langerhans cells, etc. IL-36 beta can activate M2-like macrophages and Langerhans cells; in bone marrow dendritic cells, IL-36 stimulation leads to increased production of pro-inflammatory cytokines (such as IL-1β, IL-6, IL-12, IL-23, and TNF), which may drive innate and adaptive immune responses. IL-36R is mainly expressed on naive CD4 + T cells, promoting differentiation into Th1, Th17, and promoting their proliferation and inflammation cytokine production. Moreover, IL-36 can also stimulate and activate keratinocytes, fibroblasts, and epithelial cells, indicating that both immune and non-immune cells respond to IL-36 stimulation.

Figure 5. IL-36 Functions through IL-36R in Various Immune and Non-immune Cells
IL-36 and Disease
IL-36 mediates intracellular signal transduction through IL-36R and IL-1 receptor accessory protein (IL-1RAcP). In humans, loss-of-function mutations in the gene encoding IL-36Ra lead to disordered IL-36R signaling, mainly manifested as generalized pustular psoriasis, indicating that IL-36 plays a critical role in the skin inflammation of psoriasis. In addition to being a target for skin inflammatory diseases, IL-36 can also be an effective drug target for inflammatory bowel disease (IBD), rheumatoid arthritis, and other inflammatory diseases.

Figure 6. Diagram of IL-36-related Diseases
Related Recombinant Proteins Offered by Beta LifeScience:
|
Product Name |
Cat. No. |
||
|
IL-1 SuperFamily |
IL-1 |
Human IL-1 alpha/IL -1F1 |
|
|
Biotinvlated Human IL-1 alpha/IL-1F1(C-Avi) |
|||
|
Mouse IL-1 alpha/IL -1F1 |
|||
|
Rat IL-1 alpha/IL-1F1 |
|||
|
Human IL-1 beta/IL- 1F2 |
|||
|
Mouse IL-1 beta/IL-1F2 |
|||
|
Cavia porcellus IL -1 beta/IL-1F2(C-6His) |
|||
|
Human IL -1Ra/IL-1F3 |
|||
|
Mouse IL-1Ra/IL-1F3 |
|||
|
IL-36 |
Human IL-36 alpha/IL-1F6 |
||
|
Mouse IL-36 alpha/IL-1F6 |
|||
|
Human IL-36 beta/IL-1F8(153AA) |
|||
|
Human IL-36 beta/IL-1F8(157AA) |
|||
|
Mouse IL-36 beta/IL1F8 |
|||
|
Human IL-36 gamma/IL-1F9 |
|||
|
Mouse IL -36 gamma/IL-1F9 |
|||
|
Human IL-36Ra/IL-1F5 |
|||
|
IL-18 |
[Human IL-18/IL-1F4(C-6His) |
||
|
Human IL-18/IL-1F4 |
|||
|
Mouse IL-18/IL-1F4(N-6His) |
|||
|
IL-33 |
Human IL-33/IL-1F11 |
||
|
Mouse IL-33/IL-1F11 |
|||
|
Rhesus macaque IL-33/IL-1F11(N-6His) |
|||
|
IL-37 |
Human IL-37 |
||
|
IL-1 Superfamily Receptor and Accessory Proteins |
IL-1RI |
Human IL-1RIC-Fc) |
|
|
Mouse IL-1RI(C-Fc) |
|||
|
IL-1RII |
Mouse IL-1RIK(C-Fc) |
||
|
IL-36R |
[Human IL-36R/IL-1RL2(C-6His) |
||
|
Human IL-36R/IL-1RL2(C-Fc) |
|||
|
Mouse IL-36R/IL-1RL2(C-6His) |
|||
|
Mouse IL-36R/IL-1RL2(C-Fc) |
|||
|
IL-1RAcP |
Human IL -1RAcP/IL-1R3(Q9NPH3-2,Ser21-GIn356)(C-6His) |
||
|
Human IL -1RAcP/IL-1R3(Q9NPH3-2,Ser21-GIn356)(C-Fc-6His) |
|||
|
Human IL -1RAcP/IL-1R3(09NPH3,Ser21-Glu359)(C-6His) |
|||
|
IL-18RACP |
Human IL-18RAcP/IL-1R7(C-Fc-6His) |
||
|
IL-33R |
Human I -33R/I -1RI 1(C_6His) |
||
|
Mouse IL-33R/IL-1RLI(C-6His) |
|||
|
Mouse IL -33R/IL-1RLI(C-Fc) |
Reference
[1] Dawn, Queen, Chathumadavi, et al. Function and Regulation of IL-36 Signaling in Inflammatory Diseases and Cancer Development.[J]. Frontiers in cell and developmental biology, 2019, 7:317-317.
[2] Melton E , Qiu H . Interleukin-36 Cytokine/Receptor Signaling: A New Target for Tissue Fibrosis[J]. International Journal of Molecular Sciences, 2020, 21(18).
[3] MF Neurath. IL-36 in chronic inflammation and cancer[J]. Cytokine & Growth Factor Reviews, 2020, 55.
[4] Gresnigt M S , Veerdonk F . Biology of IL-36 cytokines and their role in disease[J]. Seminars in Immunology, 2013, 25(6):458-465.














