CAR-T Cell Therapy Targets: An In-Depth Overview
Table of Contents
1. What is CAR-T therapy?
Technical Background:
In 2012, the annual meeting of the International Society for Cell Therapy pointed out that biological immune cell therapy has become the fourth means of tumor treatment besides surgery, radiotherapy, and chemotherapy, and will become a must-have means for future tumor treatment. Immune cell therapy is to collect peripheral venous blood from patients, isolate peripheral blood mononuclear cells in a GMP laboratory, and induce a large number of immune effector cells with high anti-tumor activity under the induction of various cytokines, and then through vein, intradermal Injection, intervention, etc. are reinfused into the patient's body to achieve the purpose of enhancing the patient's immune function and killing tumor cells. Commonly used immune cell therapy methods include CIK, DC, TIL, DC+CIK cells, etc.
However, when a tumor is discovered clinically, it is generally in the middle and late stage. At this time, the tumor cells in the patient's body dominate, which seriously damages the immune function of the body. In such a microenvironment of the immune system, the function of DC cells is impaired and T cells are activated. The efficiency is low, the ability to attack cancer cells is not enough, and it is not precise enough; in addition, tumor cells escape the attack of immune cells through the escape mechanism of low expression or no expression of MHC molecules. This requires the manufacture of precision-guided, precise-strike immune cell weapons to overcome the MHC-mediated tumor killing mechanism. Therefore, targeted anti-tumor cell CAR-T technology came into being.
On August 10, 2011, the authoritative magazines "Biotransformation Medicine" and "New England Journal of Medicine" reported that Carl June, a gene therapy expert at the University of Pennsylvania, cured two patients with advanced chronic lymphocytic leukemia by using the patient's modified own T cells. (blood cancer) patients, creating a new era of tumor biotherapy.
Principle of CAR-T technology:
The core of CAR-T cell therapy is to use genetic engineering technology to genetically modify the patient's T cells after collection. During genetic modification, an artificial receptor called a chimeric antigen receptor (CAR) is introduced into T cells. CAR consists of two main parts: the external antigen-binding domain and the internal signaling domain. The antigen-binding domain can recognize specific antigens expressed on cancer cells, while the signaling domain can activate T cells to kill tumor cells.
Once CAR-T cells are reinfused into the patient, they are able to recognize and bind to specific antigens present in the patient, usually on the surface of tumor cells. The antigen-binding domain of CAR enables CAR-T cells to recognize and bind to these antigens, and then the signaling domain activates T cells, causing them to release cytotoxins and pro-inflammatory factors to kill and destroy tumor cells.
Range of treatment
- Recurrent acute B-lineage lymphoblastic leukemia (relapse after remission after treatment), or refractory acute B-lineage lymphoblastic leukemia (without remission after using other anti-leukemia treatments).
- Large B-cell non-Hodgkin's lymphoma that failed two or more methods of treatment.
- CD19-positive relapsed and refractory malignant lymphoma.
- CD19 treatment failure, CD22 positive acute lymphoblastic leukemia. CAR-T therapy has also been studied in clinical trials in other anti-tumor fields, but the effect is still unclear.
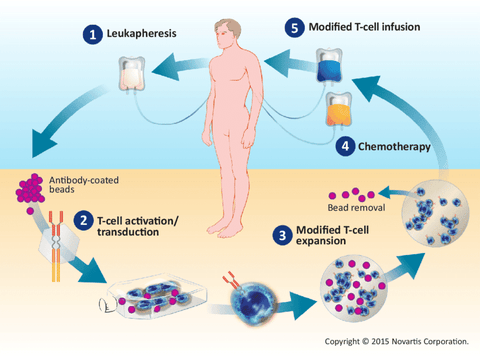
2. The Process of CAR-T Cell Therapy
- T cell collection: collect the patient's T cells through peripheral blood or bone marrow. This is usually done through a blood collection process (similar to donating blood). The collected T cells will be used for subsequent genetic modification.
- Genetic modification: The collected T cells will be genetically modified in the laboratory. This involves using genetic engineering techniques to introduce the CAR gene into T cells so that they express the CAR protein. A CAR usually consists of an antigen-binding domain, a signaling domain, and a co-stimulatory molecular domain.
- T cell expansion: The modified T cells will be expanded in the laboratory to obtain a sufficient number of CAR-T cells. This usually uses in vitro culture methods to promote T cell proliferation by providing appropriate growth factors and cell culture conditions.
- CAR-T cell injection: The expanded and prepared CAR-T cells will be reintroduced into the patient's body through intravenous injection. This is usually done in a hospital or specific treatment center and is monitored and managed by a professional medical team.
- CAR-T cells attack cancer cells: The antigen-binding domain of CAR enables CAR-T cells to recognize and bind to these antigens, and then the signaling domain activates T cells, causing them to release cytotoxins and pro-inflammatory factors, killing and destroying tumor cells.
After the process, the patient's physical reaction needs to be closely monitored, especially severe adverse reactions may occur within one to two weeks after the cells are infused into the body. The therapeutic effect on the primary disease was evaluated on the 15th and 30th day after reinfusion of CAR-T cells.
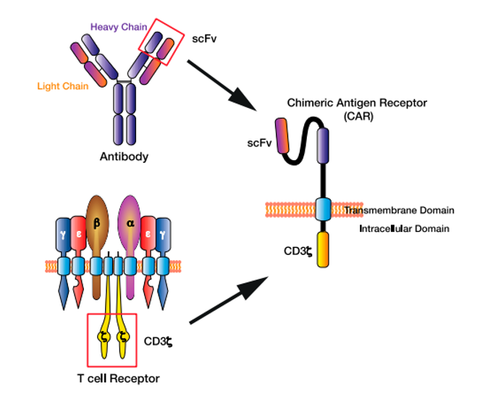
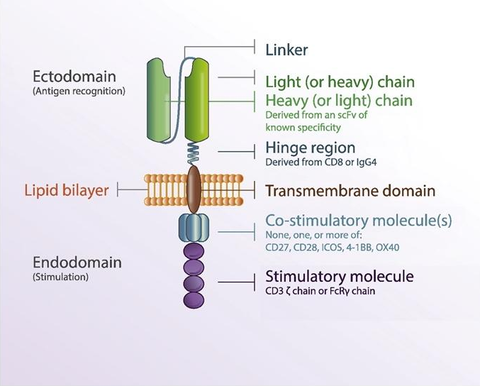
3. Structure and Generations of CAR-T Cell
The structure of CAR includes extracellular region, hinge region, transmembrane region and intracellular signal peptide region. The extracellular region is mainly the sequence of the single-chain variable region of the antigen-specific monoclonal antibody, including the variable region of the heavy chain and the variable region of the light chain, which are connected by a hinge. Its main function is to specifically recognize antigens on the surface of target cells/tumor cells in a non-MHC (Major histocompatibility complex)-restricted manner, and transmit T cell activation signals into the cells. The hinge region makes chimeric antigen receptors more flexible and promotes the combination of antigen receptors and tumor antigens; the transmembrane region is CD4, CD8, CD28 and other transmembrane protein molecules, which play a role in assisting the transmission of T cell activation signals; intracellular signals The peptide region is mainly the TCR/CD3ζ chain of T cells, which is responsible for transmitting the first activation signal into the cell. Activated T cells proliferate in large numbers and directly kill target tumor cells by secreting cytokines such as granzyme, perforin, IFN-γ and TNF-α.
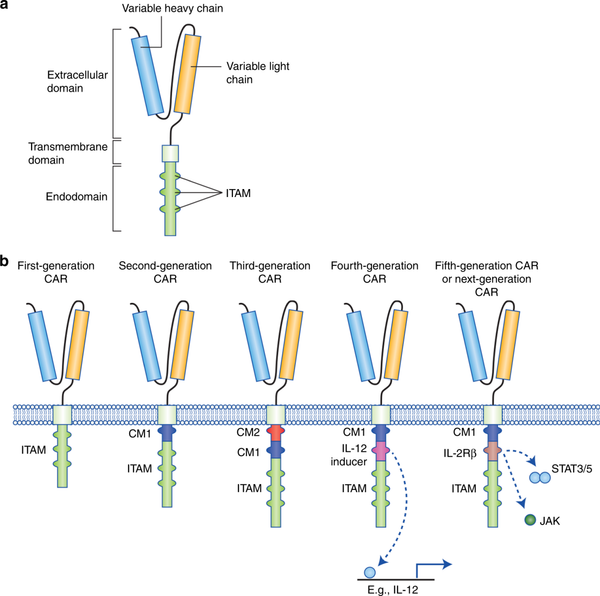
CAR-T cell therapy has been developed to the fifth generation. The following is a brief introduction to each generation of CAR-T cell therapy:
First-generation CAR-T cell therapy: The first-generation CAR-T cell therapy is the earliest version of CAR-T therapy, which consists of an antigen-binding domain and a signaling domain. Antigen-binding domains are usually the variable regions of monoclonal antibodies that recognize and bind specific tumor-associated antigens. The signaling domain is usually derived from part or all of the T cell receptor (TCR) or CD3 chain. Although the first-generation CAR-T cell therapy can recognize and kill tumor cells, its activity and persistence are limited.
Second-generation CAR-T cell therapy: In order to improve the activity and persistence of CAR-T cells, the second-generation CAR-T cell therapy adds one or more co-stimulatory molecular domains to the first generation. These co-stimulatory molecular domains can provide additional activation signals to enhance the function of CAR-T cells. Common co-stimulatory molecular domains include CD28 and 4-1BB (CD137). Compared with the first generation, the second generation of CAR-T cell therapy has achieved better therapeutic effect in clinical trials.
The third-generation CAR-T cell therapy: The third-generation CAR-T cell therapy further optimizes the combination and arrangement of co-stimulatory molecular domains on the basis of the second-generation CAR-T cell therapy. In addition, the third-generation CAR-T cell therapy can also recombine the signaling domain and costimulatory molecular domain to form a single integrated signaling domain. Such a design aims to enhance the persistence, expansion ability and anti-tumor effect of CAR-T cells.
Fourth-generation CAR-T cell therapy: The fourth-generation CAR-T cell therapy is further improved on the basis of the third generation, and molecules with regulatory functions are introduced to enhance the precision and flexibility of CAR-T cells. Common regulatory molecules include switch proteins and inhibitory proteins. These regulatory molecules can regulate the activity of CAR-T cells at the right time to avoid excessive activation or uncontrolled side effects.
The fifth generation of CAR-T cell therapy: Compared with previous generations of therapy, it has been further improved in the following aspects,First of all, the targeting is more extensive: the fifth-generation CAR-T cell therapy can target multiple tumor antigens at the same time, expanding the scope of applicable diseases and making it applicable to more types of cancer treatment. Second, the therapeutic effect is more durable: the fifth-generation CAR-T cell therapy optimizes the signal regulation and activation pathways of T cells, enhances the survival ability and persistence of T cells, and thus improves the durability of the therapeutic effect. Finally, reduce toxic and side effects: the fifth-generation CAR-T cell therapy uses specific signal regulatory molecules, so that CAR-T cells can better control the inflammatory response and cytotoxic effects when attacking tumor cells, thereby reducing the time spent on treatment toxic side effects.
4. Targets of CAR-T Cell Therapy
PE-labeled
FITC-labeled
CD19
CD19 is a cell surface protein that is a member of the B cell family of antigens. It plays an important role in the development, maturation and function of normal B cells. CD19 expression begins at the early B-cell stage and persists on the surface of mature B-cells.
CD19 participates in the activation, proliferation and antibody production of B cells by interacting with other signaling molecules. It plays an important accessory role in B cell receptor (BCR) signaling, promoting BCR signal amplification and cellular responses. In addition, CD19 also interacts with other co-stimulatory molecules and cytokines to regulate the immune response and regulatory functions of B cells.
Since CD19 is highly expressed in most B-cell malignancies, including acute lymphoblastic leukemia and non-Hodgkin's lymphoma, it has become an important molecule as a target for immunotherapy. Many clinical trials in CAR-T cell therapy use CD19 as the target antigen of CAR-T cells. By activating and guiding the patient's own T cells to recognize and kill malignant B cells expressing CD19, significant therapeutic effects have been achieved.
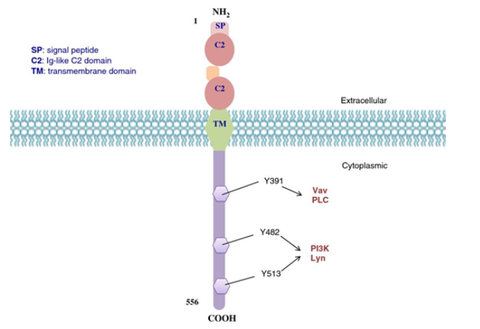
CD22
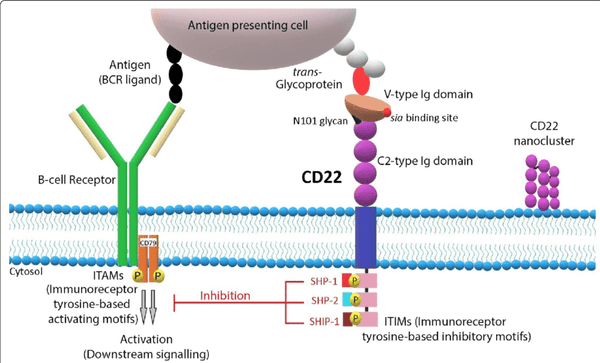
CD22 is a cell surface protein mainly expressed on mature and activated B cells of the B cell lineage. It is a receptor-type tyrosine phosphatase that regulates B cell signaling and cell adhesion.
CD22 plays an important regulatory role in B cell development and immune response. As a member of the B cell receptor complex, CD22 and B cell receptor (BCR) participate in the signal transduction and activation process of B cells. It can regulate the strength of BCR signaling through its phosphatase activity, thereby affecting the activation and proliferation of B cells. In addition, CD22 also regulates the migration and affinity of B cells through the interaction with cell adhesion molecules and other signaling molecules, and affects the immune response and regulatory functions of B cells.
Since CD22 is highly expressed in a variety of B-cell malignancies, such as B-cell acute lymphoblastic leukemia and non-Hodgkin's lymphoma, it has become an important molecule as an immunotherapy target. In recent years, CAR-T cell therapy targeting CD22 has also been widely studied and applied. By activating and guiding the patient's own T cells to recognize and kill malignant B cells expressing CD22, significant therapeutic effects have been achieved.
HER2
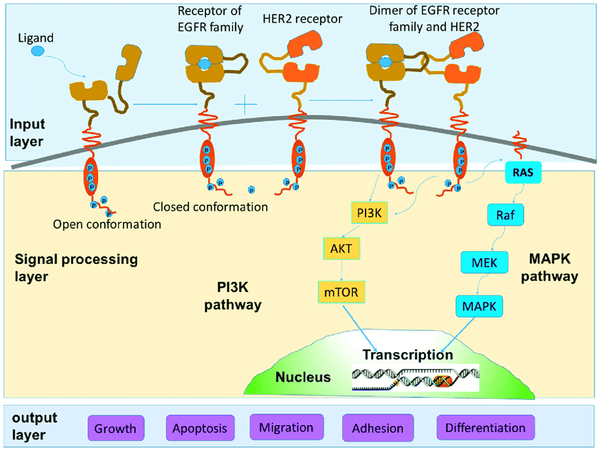
HER2 (human epidermal growth factor receptor 2) is a cell surface receptor-type tyrosine kinase also known as ERBB2 (human epidermal growth factor receptor family member 2). It is an important cell signaling molecule involved in the regulation of cell growth, proliferation and survival.
HER2 is a member of the epidermal growth factor receptor family that includes EGFR (epidermal growth factor receptor), HER2, HER3, and HER4. These receptors can bind to extracellular growth factors and activate internal kinase activities, thereby initiating a series of signal transduction pathways to regulate the physiological functions of cells. HER2 is expressed at relatively low levels in normal cells but is overexpressed in some cancers, particularly breast cancer.
Due to the overexpression of HER2 in malignant tumors such as breast cancer, it has become an important target for tumor therapy. Anti-HER2 therapies, such as the monoclonal antibody drug Trastuzumab, have been widely used in the treatment of HER2-positive breast cancer. In addition, CAR-T cell therapy has also shown potential in clinical trials targeting HER2-positive tumors.
BCMA
BCMA (B-cell maturation antigen, B-cell maturation antigen) is a cell surface protein that belongs to a member of the tumor necrosis factor (TNF) receptor superfamily. It is mainly expressed in the mature stage of B cells and is highly expressed in plasma cells.
BCMA plays an important regulatory role in the development and function of normal B cells and plasma cells. It participates in regulating the survival, proliferation and antibody production of B cells by binding to ligands such as APRIL (a proliferation-inducing ligand) and BAFF (B-cell activating factor). The BCMA signaling pathway is critical for the generation and maintenance of plasma cells as it plays a key role in regulating plasma cell proliferation and longevity.
Since BCMA is highly expressed in B-cell malignancies such as plasmacytoma, it has become an important target for immunotherapy. Recently, CAR-T cell therapy targeting BCMA has achieved remarkable clinical results. CAR-T cells carry BCMA-specific receptors to recognize and kill BCMA-expressing malignant B cells, thereby achieving therapeutic effects on plasmacytoma.

5. CAR-T Cell Therapy Clinical Trial and Side Effect
Approximately one-third of ongoing CAR-T clinical trials are evaluating use in solid tumors; CAR-T therapies targeting solid tumors have yet to demonstrate similar clinical responses to hematological cancers; the current focus of the CAR-T field is development Methods and technologies for overcoming the immunosuppressive tumor environment, tumor accessibility and invasion, and CAR-T function optimization to improve the treatment of solid tumors.
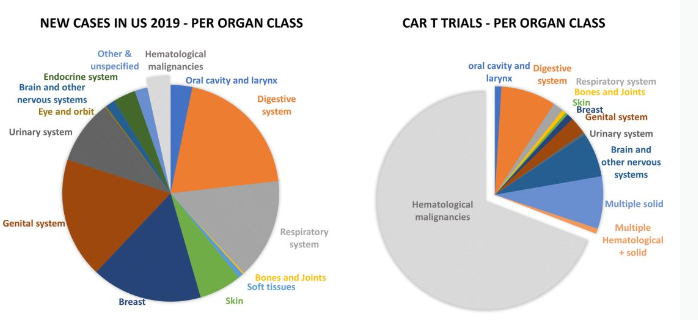
Although CAR-T therapy is considered a promising treatment, it may also cause some side effects.
Overexpansion of CAR-T cells: Sometimes CAR-T cells may overexpand, resulting in uncontrolled cell numbers. This condition may lead to increased severity of cytokine release syndrome (CRS), as well as increased hematologic toxicity.
Severe cytokine release syndrome (CRS): CRS is one of the most common side effects of CAR-T cell therapy. In some cases, CRS can be severe, causing high fever, low blood pressure, shortness of breath, and multiple organ dysfunction. In extreme cases, CRS can lead to death.
Neurotoxicity: CAR-T cell therapy can sometimes lead to severe neurotoxicity, including increased intracranial pressure, cerebral edema, and neurological dysfunction. These reactions can lead to coma, seizures, and long-term cognitive and movement impairments.
Immune responses and autoimmune diseases: CAR-T cell therapy may cause abnormal reactions in the patient's immune system, including the development of autoimmune diseases. This can lead to arthritis, Sjogren's syndrome, thyroid dysfunction, and other autoimmune diseases in patients.
It should be emphasized that although there are these negative cases of CAR-T cell therapy, this is only the case of a small number of patients, and these cases do not mean that every patient receiving CAR-T cell therapy will experience the same problems. The medical team closely monitors patients while administering CAR-T cell therapy and takes steps to manage and mitigate possible side effects. Patients should fully discuss the risks and benefits of CAR-T cell therapy with their physicians and make an informed decision.
Reference
[1] Hughes-Parry HE, Cross RS, Jenkins MR. The Evolving Protein Engineering in the Design of Chimeric Antigen Receptor T Cells. Int J Mol Sci. 2019;21(1):204. Published 2019 Dec 27. doi:10.3390/ijms21010204
[2] McGuirk J, Waller EK, Qayed M, et al. Building blocks for institutional preparation of CTL019 delivery. Cytotherapy. 2017;19(9):1015-1024. doi:10.1016/j.jcyt.2017.06.001
[3] Gill S, Maus MV, Porter DL. Chimeric antigen receptor T cell therapy: 25years in the making. Blood Rev. 2016;30(3):157-167. doi:10.1016/j.blre.2015.10.003
[4] Tokarew, N., Ogonek, J., Endres, S. et al. Teaching an old dog new tricks: next-generation CAR T cells. Br J Cancer 120, 26–37 (2019). https://doi.org/10.1038/s41416-018-0325-1
[5] Wang K, Wei G, Liu D. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol. 2012;1(1):36. Published 2012 Nov 29. doi:10.1186/2162-3619-1-36 https://pubmed.ncbi.nlm.nih.gov/23210908/
[6] Aujla, A., Aujla, R. & Liu, D. Inotuzumab ozogamicin in clinical development for acute lymphoblastic leukemia and non-Hodgkin lymphoma. Biomark Res 7, 9 (2019). https://doi.org/10.1186/s40364-019-0160-4
[7] Lv Q, Meng Z, Yu Y, et al. Molecular Mechanisms and Translational Therapies for Human Epidermal Receptor 2 Positive Breast Cancer. Int J Mol Sci. 2016;17(12):2095. Published 2016 Dec 14. doi:10.3390/ijms17122095
[8] Guo R, Lu W, Zhang Y, Cao X, Jin X, Zhao M. Targeting BCMA to Treat Multiple Myeloma: Updates From the 2021 ASH Annual Meeting. Front Immunol. 2022;13:839097. Published 2022 Mar 7. doi:10.3389/fimmu.2022.839097
[9] Springuel L, Lonez C, Alexandre B, et al. Chimeric Antigen Receptor-T Cells for Targeting Solid Tumors: Current Challenges and Existing Strategies. BioDrugs. 2019;33(5):515-537. doi:10.1007/s40259-019-00368-z










