The Role of MMP-3: Insights into Disease Progression and Therapeutic Potential
The MMP Family
Matrix metalloproteinases (MMPs) are a family of enzymes belonging to the metzincin superfamily. There are 23 different types of secreted or membrane-anchored endopeptidases in the human body. MMPs are capable of degrading ECM proteins such as collagen, gelatin, laminin, fibronectin, fibrinogen, elastin, and proteoglycans. They were once considered regulators of the extracellular matrix. MMPs have multiple structural domains, including a signal peptide (SP), propeptide domain (Pro), catalytic domain (Catalytic), variable linker "hinge" region (Hinge), and a hemopexin domain (Hemopexin). These five domains are shared among MMPs [1].
MMPs are classified into different subfamilies based on their protein structure and substrate specificity. These subfamilies include collagenases (MMP-1, MMP-8, MMP-13), gelatinases (MMP-2, MMP-9), stromelysins (MMP-3, MMP-10, MMP-11), matrilysins (MMP-7, MMP-26), membrane-type MMPs (MMP-14, MMP-15, MMP-16, MMP-17, MMP-24, MMP-25), and other unclassified family members (MMP-12, MMP-19, MMP-20, MMP-21, MMP-23, MMP-27, MMP-28) based on their protein structure and substrate specificity.
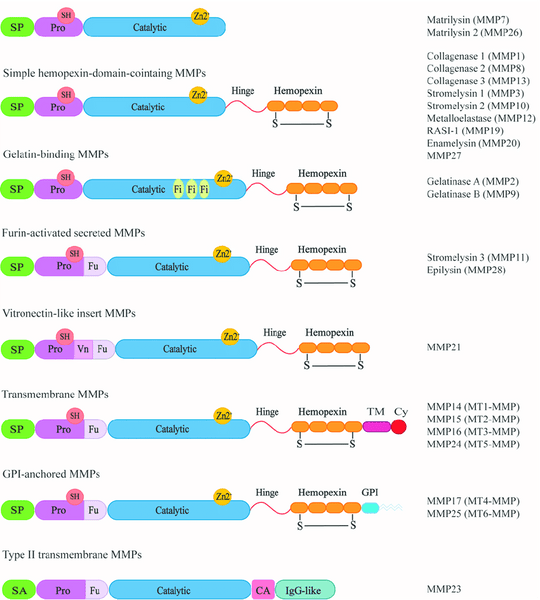
Collagenases recognize substrates and degrade fibrillar collagen through the hemopexin structural domain. Gelatinases can digest various ECM components, such as type I and type IV collagen. Stromelysins have a domain arrangement similar to collagenases but cannot cleave type I collagen fibrils. Matrilysins lack the hemopexin domain and can degrade type IV collagen. Membrane-type MMPs have an additional C-terminal transmembrane domain and a short cytoplasmic tail. Among them, MMP-14 and MMP-16 can degrade type I collagen [2].
Introduction - MMP-3
MMP-3, also known as stromelysin-1, is a key member of the MMP family. It is synthesized in cells as a proenzyme form (pro-MMP-3) and consists of 477 amino acids. The relative molecular weight of MMP-3 is approximately 57,000, with an active portion of around 45,000. Its structure can be divided into three parts:
(1) N-terminal propeptide region: It contains a signal peptide composed of 17 amino acid residues, which guides the newly synthesized MMP-3 towards the cell membrane.
(2) N-terminal catalytic domain: Connected to the N-terminal propeptide region by a hinge region, this domain contains a Zn2+ binding motif, serving as the active site of the entire proteinase.
(3) C-terminal domain: Its amino acid sequence is similar to that of heme-binding proteins and is the site where MMP-3 binds to substrates [4]. Typically, MMP-3 exists in at least three forms: the proenzyme form (pro-MMP-3), the active form (active MMP-3), and the complex formed with tissue inhibitor of metalloproteinase (TIMP) known as the MMP-3/TIMP complex [3].
Clinical Applications of MMP-3
MMP-3 and Rheumatoid Arthritis (RA)
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by synovial cell proliferation and infiltration of lymphocytes, leading to destruction of joint cartilage and bone erosion. Previous studies have shown that MMP-3 levels in the serum of RA patients are significantly higher compared to healthy individuals. In addition to being used as an auxiliary marker, MMP-3 can also serve as a prognostic monitoring marker and be used to assess the efficacy of drug treatments. A comparison was conducted between the serum MMP-3 levels of 45 RA patients and 20 healthy individuals undergoing physical examinations. The serum MMP-3 levels of RA patients in the active phase before treatment were significantly higher than those of the normal control group. After treatment, MMP-3 levels decreased, with a significant difference observed in those who experienced symptom relief. Furthermore, MMP-3 is a reliable biomarker for assessing disease activity, imaging monitoring, predicting disease outcomes, and treatment response in rheumatoid arthritis [4].
MMP-3 and Ankylosing Spondylitis (AS)
AS is a chronic inflammatory disease that primarily affects the spine and sacroiliac joints. Currently, HLA-B27, MRI, and the Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) are commonly used clinical criteria for diagnosing AS and assessing its activity. Increasing evidence suggests a close association between MMP-3 and AS.
By comparing the levels of MMP-3 in the serum of 175 AS patients and 95 normal healthy individuals, it was found that MMP-3 is elevated in the serum of AS patients. It not only reflects joint bone destruction in AS but also provides a better reflection of disease activity. Therefore, it can serve as a serological marker indicating disease progression and improvement in AS, in addition to ESR and CRP [4].
MMP-3 and Osteoarthritis
OA, also known as osteoarthritis, is a common form of arthritis characterized by the loss of joint cartilage, leading to joint space narrowing, increased friction in the joints, persistent pain, and functional impairment. Previous studies have indicated that the longer the disease duration and the more severe the symptoms in OA patients, the more significant the cartilage damage and synovial fibrosis. The levels of MMP-3, MMP-13, and IL-17 in the synovium are also higher, showing a positive correlation between these factors. MMP-3 has shown good predictive accuracy in the treatment of OA.
MMP-3 and Lupus Erythematosus (SLE)
Systemic lupus erythematosus (SLE) is an autoimmune disease that can affect multiple organs, and its exact pathogenesis is not yet fully understood. It may be related to factors such as infection, endocrine dysfunction, and immune dysregulation. MMP-3 is involved in the development of SLE by degrading the vascular matrix components in SLE patients' blood vessel walls.
In recent years, further research on MMP-3 has revealed its association with conditions such as glioma, ocular tumors, breast cancer, gastric cancer, non-small cell lung cancer, liver cancer, and even COVID-19. However, the superiority of MMP-3 is increasingly prominent in early diagnosis, disease activity monitoring, treatment guidance, and prognosis assessment in rheumatoid arthritis (RA). It is believed that in the near future, MMP-3 will be incorporated into the routine assessment, monitoring, and treatment of RA patients.
BetaLifeScience provides high-quality MMP-3 antigens and antibodies that have been validated on LF (Lateral Flow) and CLIA (Chemiluminescent Immunoassay) platforms, contributing to the development of in vitro diagnostic test kits.
- High affinity reaching pM level
- Strong stability, validated through freeze-thaw and accelerated stability testing
- Verified by TRFIA/CLIA, with a linear range of 1~1000 ng/mL.
Recommended MMP-3 Products
|
Cat. # |
Product Name |
References
[1] Madzharova E, Kastl P, Sabino F, Auf dem Keller U. Post-Translational Modification-Dependent Activity of Matrix Metalloproteinases. Int J Mol Sci. 2019 Jun 24;20(12):3077. doi: 10.3390/ijms20123077. PMID: 31238509; PMCID: PMC6627178.
[2] Pittayapruek P, Meephansan J, Prapapan O, Komine M, Ohtsuki M. Role of Matrix Metalloproteinases in Photoaging and Photocarcinogenesis. Int J Mol Sci. 2016 Jun 2;17(6):868. doi: 10.3390/ijms17060868. PMID: 27271600; PMCID: PMC4926402.
[3] Wan J, Zhang G, Li X, Qiu X, Ouyang J, Dai J, Min S. Matrix Metalloproteinase 3: A Promoting and Destabilizing Factor in the Pathogenesis of Disease and Cell Differentiation. Front Physiol. 2021 Jul 2;12:663978. doi: 10.3389/fphys.2021.663978. PMID: 34276395; PMCID: PMC8283010.
[4] Lerner A, Neidhöfer S, Reuter S, Matthias T. MMP3 is a reliable marker for disease activity, radiological monitoring, disease outcome predictability, and therapeutic response in rheumatoid arthritis. Best Pract Res Clin Rheumatol. 2018 Aug;32(4):550-562. doi: 10.1016/j.berh.2019.01.006. Epub 2019 Feb 14. PMID: 31174824.














