Tumor Necrosis Factor (TNF) Superfamily
Introduction of Tumor Necrosis Factor (TNF) Family
In 1975, E.A. Carswell et al. found that after the mice inoculated with BCG were injected with bacterial lipopolysaccharide, a substance that could cause hemorrhagic necrosis of various tumors appeared in the serum, and they named it tumor necrosis factor (TNF). In the 1980s, it was discovered that it played an important role in wasting disease, also known as cachectic factor. TNF is mainly produced by activated macrophages, NK cells and T lymphocytes. In 1985, Shalaby named the TNF produced by macrophages as TNF-α, and the lymphotoxin (LT) produced by T lymphocytes as TNF-β. Although TNF-α and TNF-β have only about 30% homology, they share a common receptor. The biological activity of TNFα accounts for 70% to 95% of the total activity of TNF, so the TNF that is often said now refers to TNF-α. The cloning of the TNF gene in 1984 ushered in the era of clinical trials. It was the first cytokine used in tumor biotherapy, but it is currently only used for local treatment because of its lack of targeting and serious side effects.
Characteristics and Regulation of the TNF Gene
The TNF-α gene in humans was successfully cloned in 1985 and is located at 6p21.4. It spans approximately 3.6 kbp and consists of 4 exons and 3 introns. It is closely linked with the major histocompatibility complex (MHC) gene. Within the MHC3 gene region, between the HLA-B and HLA-C2 loci, there are two genes: TNFA and TNFB, which encode TNFα and TNFβ, respectively. Single nucleotide polymorphisms (SNPs) at positions 238 and 308 in the promoter region are known to regulate the transcription level of TNF and are associated with susceptibility to chronic hepatitis B, autoimmune diseases, insulin resistance, tumors, and other diseases. The mRNA encoded by the TNF gene is approximately 1.7 kbp, and its 3'untranslated region contains a conserved TTATTTAT sequence known as the AU-rich element (ARE).
Phorbol ester is an inducer of TNF and can stimulate the transcription of TNF through a short sequence located near the TATAA box in the promoter region. This induction mechanism involves the binding of specific transcription factors to the promoter region, facilitating the activation of TNF gene expression.
Structural and Functional Properties of TNF Proteins
-
TNF-α:
-
The human TNF-α precursor consists of 233 amino acids (26 kDa) and contains a signal peptide composed of 76 amino acid residues.
- TNF-converting enzyme TACE processes the precursor, removing the signal peptide and generating a mature form of TNF-α with 157 amino acid residues (17 kDa).
- TNF-α lacks glycosylation sites and has an intramolecular disulfide bond formed by cysteines at positions 69 and 101.
- Human TNF-α shares 79% amino acid homology with mouse TNF-α, suggesting no significant species specificity in its biological role.
- Genetic engineering techniques have been employed to create variants of TNF-α with improved biological activity and anti-tumor effects.
-
Natural TNF-α and β exert their biological effects as homologous trimers.
-
TNF-β:
-
The human TNF-β molecule consists of 205 amino acid residues, including a signal peptide of 34 amino acid residues.
-
The mature form of TNF-β is composed of 171 amino acid residues, with a molecular weight of 25 kDa.
-
Homology and Structure:
-
Human TNF-β and TNF-α share 56% DNA sequence homology and 36% amino acid homology.
- X-ray crystallography studies confirm that TNF proteins exist as compact trimers, composed of three identical monomer subunits.
- The monomer subunits have a wedge-shaped structure and are folded by β-sheets to form a β-sandwich structure.
TNF Receptors: Types, Structure, and Functions
TNF Receptor (TNFR) Characteristics
TNFR can be classified into two types: Type I TNF-R (also known as TNFR1, CD120a, p55) and Type II TNFR (also known as TNFR2, CD120b, p75).
Type I TNFR:
- Consists of 439 amino acid residues and has a molecular weight of 55 kDa.
- Its corresponding mRNA is approximately 4.5 kbp in length.
- Type I TNFR is expressed on various cell types and primarily contributes to cytolytic activity.
Type II TNFR:
- Comprises 426 amino acid residues and has a molecular weight of 75 kDa.
- Its corresponding mRNA is approximately 3 kbp in length.
- Type II TNFR is selectively expressed on immune and endothelial cells and is involved in signal transmission and T cell proliferation.
Both types of TNFR are glycoproteins that consist of three regions: extracellular, transmembrane, and intracellular. Although the extracellular region shares 28% homology, the cytoplasmic region lacks homology, indicating its involvement in mediating distinct signal transduction pathways. Research confirms that TNF exerts its biological activity mainly through interaction with TNF-R1. The binding of TNF to TNF-R1 induces TNF-R1 aggregation and the release of the silencer of death domain (SODD), enabling the binding of TNF-R1 to TRADD and subsequent recruitment of adapter proteins such as RIP, TRAF-2, and FADD. These adapter proteins further recruit other important proteins involved in signal transduction.
TNFR2 possesses a different structure and function, lacking a death domain. Consequently, it cannot directly promote apoptosis. However, TNFR2 can influence programmed cell death (PCD) by activating the NF-κB and JNK pathways or inhibiting TRAF-2.
Expression and Function of TNFRs
TNFRs are present on the surface of various normal and tumor cells, with an average of 500-5000 receptors per cell. The number and affinity of TNFRs on different cell surfaces do not necessarily correlate with the sensitivity of cells to TNF-α.
TNF-binding protein (TNF-BP) represents a soluble form of TNFR, which includes two types: sTNFRⅠ (TNF-BPⅠ) and sTNFRⅡ (TNF-BPⅡ). These soluble receptors are believed to regulate the cytokine network by limiting TNF activity or stabilizing TNF. TNF-BP has been found in the urine of febrile patients and in the blood and urine of patients with chronic renal insufficiency. It can specifically bind to TNF, inhibiting its cytotoxic activity and IL-1 production. Moreover, TNF-BP levels can increase in various conditions such as inflammation, endotoxemia, meningococcal infection, SLE, HIV infection, renal insufficiency, and tumors. Soluble TNFR has been effective in alleviating the pathological changes associated with adjuvant arthritis and septic shock.
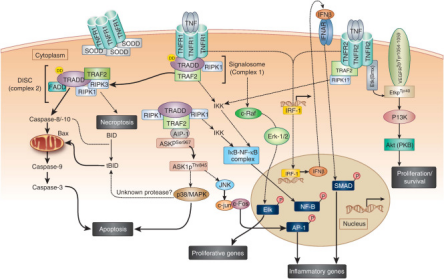
The TNF Signaling Pathway: Regulation and Therapeutic Implications
TNF Signaling Pathway
The TNF (Tumor Necrosis Factor) signaling pathway encompasses a complex cascade of molecular events involving multiple signaling molecules and pathways. This intricate network regulates various physiological and pathological processes within cells. The following steps outline the key components of the TNF signaling pathway:
- Binding to Receptors: TNF binds to its receptor TNFR (Tumor Necrosis Factor Receptor), forming a TNF-TNFR complex. Additionally, other receptors such as TNFR2 also belong to the TNF receptor family.
- Receptor Aggregation: Formation of TNF-TNFR complexes triggers the aggregation of receptors and the formation of aggregation domains.
- Activation of Transmitters: Receptor aggregation leads to the release of activating transmitters, including intracellular molecules such as TRADD (TNFR-associated death domain protein), TRAF (TNFR-associated factor), and RIP (receptor subcellular protein).
- Signaling: Transmitter molecules activate downstream signaling pathways, including the well-known NF-κB (nuclear factor-κB) pathway, MAPK (mitogen-activated protein kinase) pathway, and JNK (c-Jun N-terminal kinase) pathway.
- Gene Transcription: Activated signaling pathways modulate the transcription of specific genes by influencing the activity and nuclear translocation of transcription factors. Notably, NF-κB plays a critical role, as it enters the nucleus upon activation and regulates the transcription of various inflammation-related genes.
- Biological Effects: Signal transmission through the TNF pathway ultimately elicits a range of biological effects both within and outside cells. These effects include the initiation of inflammatory responses, apoptosis, cell proliferation, cell migration, and immune regulation.
Significance and Therapeutic Implications
Activation of the TNF signaling pathway holds paramount importance in physiological and pathological processes such as inflammation, immune regulation, cell survival, and cell death. Dysregulated activation of this pathway is associated with the development of diverse diseases, including inflammatory diseases, autoimmune disorders, cancer, and neurodegenerative conditions. Consequently, the TNF signaling pathway has become a crucial therapeutic target, and anti-TNF drugs and other agents that target this pathway have gained widespread usage in clinical treatments.
Clinical Applications of the TNF (Tumor Necrosis Factor) Family
- Treatment of Autoimmune Diseases: Autoimmune diseases, characterized by the immune system attacking its own tissues, include conditions like rheumatoid arthritis, ankylosing spondylitis, and psoriasis. Anti-TNF therapy has emerged as the first-line treatment for these diseases. It effectively reduces inflammation, alleviates symptoms, and improves the overall quality of life for patients.
- Cancer Treatment: TNF production by certain tumor cells promotes tumor growth, metastasis, and immune evasion by stimulating inflammatory responses and angiogenesis. Hence, blocking the action of TNF can be a valuable strategy in cancer treatment. By inhibiting TNF, tumor growth and spread can be hindered, while also enhancing the immune system's ability to recognize and target tumors.
- Immunomodulation: Members of the TNF family play a crucial role in immune system regulation. Anti-TNF therapy can modulate immune function and impact cell-mediated immune responses. This treatment approach is useful in controlling an overactive immune system, such as in immunosuppression following organ transplantation and the management of autoimmune diseases.
- Treatment of Neurodegenerative Diseases: TNF also exerts significant influence in the nervous system and is implicated in the development of neurodegenerative diseases. Inhibiting TNF action presents a potential therapeutic avenue for conditions like Alzheimer's disease and Parkinson's disease. By targeting TNF, the progression of neurodegenerative diseases can be mitigated.
Summary
In summary, TNF and its receptors play important regulatory roles in immune, inflammatory and disease processes. Understanding its signaling mechanism and biological function will help to deeply understand the occurrence and development of related diseases, and provide new strategies and drug targets for the treatment of related diseases.
References:
[1] Watts, & Tania, H. . (2005). Tnf/tnfr family members in costimulation of t cell responses. Annual Review of Immunology, 23(1), 23.
[2] Grzela, T. , M Ołdak, J Jóźwiak, & Malejczyk, J. . (1999). [induction of apoptosis by receptors for factors from the tnf family]. Postpy Higieny I Medycyny Dowiadczalnej, 53(2), 351.
[3] Kovalenko, A. . (2011). Advances in tnf family research. Advances in Experimental Medicine & Biology, 691.
[4] Al-Lamki RS, Mayadas TN. TNF receptors: signaling pathways and contribution to renal dysfunction. Kidney Int. 2015;87(2):281-296. doi:10.1038/ki.2014.285










