Interferons (IFNs) and Receptors Overview
Interferons (IFNs)
Interferon (IFN) is a group of cytokines produced by monocytes and lymphocytes with similar structures and functions. They have broad-spectrum anti-virus, affect cell growth, and differentiate, regulate immune function and other biological activities. They were originally discovered by Alick Issacs and Jean Lindenmann in 1957 when they studied the phenomenon of virus interference. The biological effect of IFN needs to bind to the corresponding signal receptor, that is, the interferon receptor (IFNR). IFN receptors are found on monocytes, macrophages, T lymphocytes, glial cells and neurons. Interferon receptors are complex structures consisting of many different transmembrane polypeptides and a large number of protein tyrosine kinases capable of transient interactions with other proteins. According to the classification of IFN, interferon receptors can be divided into type I interferon receptors, type II interferon receptors and type III interferon receptors.
Interferon receptor
1. Type I interferon receptor
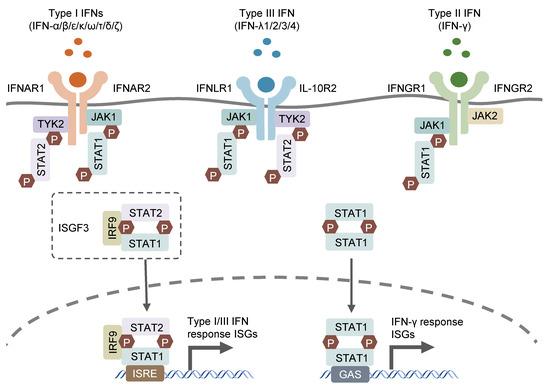
The human type I interferon receptor gene is located on chromosome 21, distributed on the cell surface, and contains at least two subunits named α (IFNAR1) and β (IFNAR2), which belong to the type II cytokine receptor family. The α subunit has only one form, while the β subunit has both soluble and transmembrane forms. IFNAR2 (β subsingle) has three types produced by alternative splicing of the same gene: IFNAR2a (βs short form), IFNAR2b (β soluble form) and IFNAR2c (βL long form). The βs short form and the βL long form are distinguished by the first 16 amino acids of the extracellular and transmembrane domains and the cytoplasmic domain. The soluble form was generated by inserting a stop codon after amino acid 236. The long β subunit (βL) is 515 amino acid residues long and the initial form for signal generation.
Type I interferons are mainly secreted by innate immune cells, mainly IFN-α and IFN-β. IFN β is mainly produced by human fibroblasts; IFN-α is mainly produced by monocyte-macrophages, and B cells and fibroblasts can also synthesize IFN-α. IFN-α/β is widely distributed, including monocyte-macrophages, polymorphonuclear leukocytes, B cells, T cells, platelets, epithelial cells, endothelial cells and tumor cells. IFNα and IFNβ function by binding to cell surface receptors IFNAR1 and IFNAR2, and TYK2 and JAK1 in protein tyrosine kinases are associated with IFNAR1 and IFNAR2, respectively. After kinase activation, the transcription factors STAT1 and STAT2 in the cytoplasm will be activated, and the two will aggregate into the nucleus to assist some effector genes downstream of IRF9 transcription.
2. Type II interferon receptor
Type II interferon receptors (IFNGR) are divided into a ligand-adsorbed chain (IFNGR1) and an accessory chain (IFNGR2), both of which belong to the cytokine class II receptor superfamily. IFNGR complexes are widely distributed in various cells and tissues. IFNGR1 binds ligands with high specificity, but type II interferons (i.e., IFNγ) can induce IFNγ-related signaling and activity only in the presence of IFNGR2.
IFNγ is mainly produced by activated T cells and NK cells, and is a kind of lymphokine. IFN-γ can exist in the form connected to the extracellular matrix, so it can control cell growth by neighbors, and it can be distributed on the surface of almost all cells except mature red blood cells. IFNγ and its receptors signal through the JAK/STAT pathway, resulting in the formation of phosphorylated STAT1 homodimers (also known as IFNγ activators), also known as STAT1. Activated IFNγ activator translocates to the nucleus and binds the γ-activating sequence (GAS) in the upstream promoter region of interferon-inducible genes. IFN-γ plays an important role in innate immunity and adaptive immunity against viruses, certain bacteria and protozoan infections. IFN-γ is an important activator of macrophages and an inducer of type II major histocompatibility complex (MHC II) expression. Abnormal expression of IFN-γ is associated with many autoinflammatory and autoimmune diseases. In addition to directly inhibiting virus replication, the importance of IFN-γ to the immune system is more reflected in its immune stimulation and immune regulation functions.
3. Type Type III interferon receptors
Type III interferon receptors are heterodimeric IFNLR1/IL10R2 complexes that specifically bind to type III interferon. The type III interferon family is a family discovered in recent years, including IFNλ-1 (also known as IL29), IFNλ-2 (IL28A), IFNλ3 (IL-28B) and IFNλ4 four members. It is produced by antigen presenting cells and epithelial cells. Type III interferons exhibit similar functions to type I interferons. Furthermore, they are the main protectors of the barrier integrity of mucosal sites. At mucosal sites they stimulate pathogen clearance while suppressing inflammation to maintain barrier integrity.
Interferon Signaling Pathway
- Activation of receptors: Interferon binds to the corresponding receptors, such as IFN-α and IFN-β to IFN-α/β receptors, and IFN-γ to IFN-γ receptors, thereby activating the initiation of signal transduction start step.
- JAK-STAT signaling pathway: The activation of interferon receptors leads to the activation of receptor-associated kinases (JAK). Activated JAKs further phosphorylate the receptors, thereby providing binding sites for signal transducers and activators of transcription (STAT) proteins to bind. Phosphorylated STAT proteins form dimers or trimers, migrate to the nucleus and bind to interferon response elements (ISREs), initiating the transcription of interferon-responsive genes.
- Expression of ISGs: Activation of the interferon signaling pathway leads to the expression of a variety of interferon-stimulated genes (ISGs), including genes with antiviral and immune regulatory functions. The expression of these ISGs can enhance the antiviral ability of cells, inhibit virus replication, and promote the activation and apoptosis of immune cells.
- Regulation of other signaling pathways: The interferon signaling pathway can also interact with other signaling pathways, such as NF-κB, MAPK and PI3K signaling pathways. Through cross-regulation and interaction, the interferon signaling pathway can regulate the development, differentiation and function of immune cells.
The clinical significance of interferon
- Antiviral treatment: Interferon has antiviral activity and can inhibit virus replication and transmission, so it is widely used to treat a variety of 1. viral infections, such as hepatitis B virus, hepatitis C virus, human immunodeficiency virus (HIV), etc.
- Tumor treatment: Interferon has anti-tumor activity, can inhibit the proliferation and spread of tumor cells, induce tumor cell apoptosis, and enhance the anti-tumor effect of immune cells. Therefore, interferon is used to treat various tumors, such as chronic myelogenous leukemia, melanoma, renal cell carcinoma, etc.
- Immunomodulatory therapy: Interferon can regulate the function of the immune system, enhance the activity of immune cells and immune response, and regulate inflammatory response. Therefore, interferon is used to treat various immune-related diseases, such as multiple sclerosis, rheumatoid arthritis, etc.
- Adjuvant therapy of anti-tumor immunotherapy: Interferon can enhance the ability of immune cells to attack tumors and improve the efficacy of anti-tumor immunotherapy. Therefore, interferon is often used in combination with other anti-tumor treatments, such as chemotherapy, radiotherapy and immunotherapy, to enhance the therapeutic effect.
Summarize
In summary, interferon plays a key role in immune response and antiviral defense. It can activate the antiviral defense mechanism of cells, enhance the activity of immune cells, regulate inflammatory response, inhibit virus replication and cell proliferation. Therefore, members of the interferon family are widely used clinically to treat various diseases such as viral infections, tumors, and immune-related diseases.
References:
[1] Goodbourn, S. , Didcock, L. , & Randall, R. E. . (2000). Interferons: cell signalling, immune modulation, antiviral response and virus countermeasures. Journal of General Virology, 81(10), 2341-2364.
[2] Pestka, S. , Krause, C. D. , & Walter, M. R. . (2010). Interferons, interferon-like cytokines, and their receptors. Immunological Reviews, 202(1), 8-32.
[3] Berenguer, J. , RodrÃguez-Castellano E, Carrero, A. , Von Wichmann, M. A. , Montero, M. , & Galindo, M. J. , et al. (2017). Eradication of hepatitis c virus and non‐liver‐related non–acquired immune deficiency syndrome–related events in human immunodeficiency virus/hepatitis c virus coinfection. Hepatology, 66(2), 344-356.
[4] Deng S, Graham ML, Chen X-M. The Complexity of Interferon Signaling in Host Defense against Protozoan Parasite Infection. Pathogens. 2023; 12(2):319. https://doi.org/10.3390/pathogens12020319









