EGFR Protein: A Key Player in Cancer and Beyond
Basic Information of EGFR Protein
EGFR (Epidermal Growth Factor Receptor) is a critical membrane receptor protein that plays a pivotal role in various physiological processes, including cell growth, proliferation, differentiation, and survival. Belonging to the tyrosine kinase receptor family, EGFR is activated upon binding with external epidermal growth factor (EGF) and similar factors, functioning as a key regulator of cellular and tissue homeostasis.
Under normal physiological conditions, EGFR maintains a delicate balance in cellular activities and tissue functioning. However, in certain instances, abnormal activation of EGFR is associated with the onset and progression of various diseases. Notably, in cancer, EGFR can be overexpressed or mutated, leading to aberrant cell proliferation and metastasis, thus facilitating tumor initiation and advancement.
Due to its significant involvement in cancer development, EGFR has emerged as a crucial target for anticancer therapies. Researchers focus on understanding EGFR's molecular pathways to develop targeted treatments that effectively inhibit its overactivation and combat cancer growth.
So far, six EGFR ligands have been identified, including epidermal growth factor (EGF), transforming growth factor alpha (TGFA), amphiregulin (AR), betacelluin (BTC), heparin-binding EGF (HBEGF), and epiregulin (EPR). Among these ligands, EGF and TGF-alpha hold particular significance as the most crucial ones in EGFR signaling.
EGFR plays a pivotal role in signal transduction, cell proliferation, differentiation, and various regulatory mechanisms. However, in human tumors, it is frequently overexpressed and dysregulated, making it a prominent focus in tumor diagnosis and treatment research.
The Structure of EGFR Protein
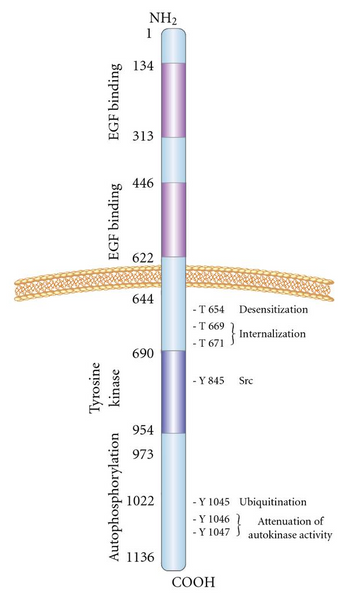
The hEGFR gene is located on human chromosome 7p, and its encoded product is a single-strand transmembrane protein containing 1210 amino acid residues with a molecular weight of 134 kDa. The peptide fragment is further glycosylated at the 11th to 12th aspartic acid residue and its molecular weight increases to 170 kDa, becoming a mature EGFR.
The EGFR protein is made up of several different domains, each with a specific function:
- Extracellular Domain: The extracellular domain of EGFR is located on the outer surface of the cell membrane and contains four subdomains (I-IV). Subdomains I and III are involved in ligand binding, and they are responsible for recognizing and binding to the various EGFR ligands, such as EGF and TGF-alpha. Ligand binding induces a conformational change in the receptor.
- Transmembrane Domain: The transmembrane domain is a hydrophobic segment of the EGFR protein that spans the cell membrane. It anchors the receptor to the cell surface and plays a critical role in signal transmission from the extracellular domain to the intracellular domain.
- Intracellular Domain: The intracellular domain of EGFR is located inside the cell and contains the kinase domain, which has tyrosine kinase activity. Upon ligand binding, the receptor undergoes dimerization, where two EGFR molecules come together, leading to the activation of the kinase domains. This results in the autophosphorylation of specific tyrosine residues within the intracellular domain, creating docking sites for various downstream signaling molecules.
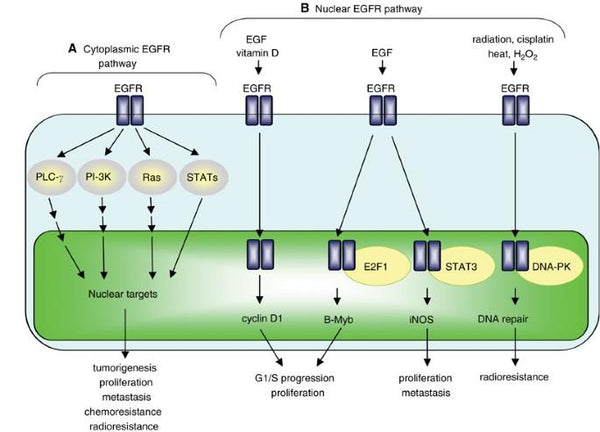
EGFR Protein Signaling Pathway
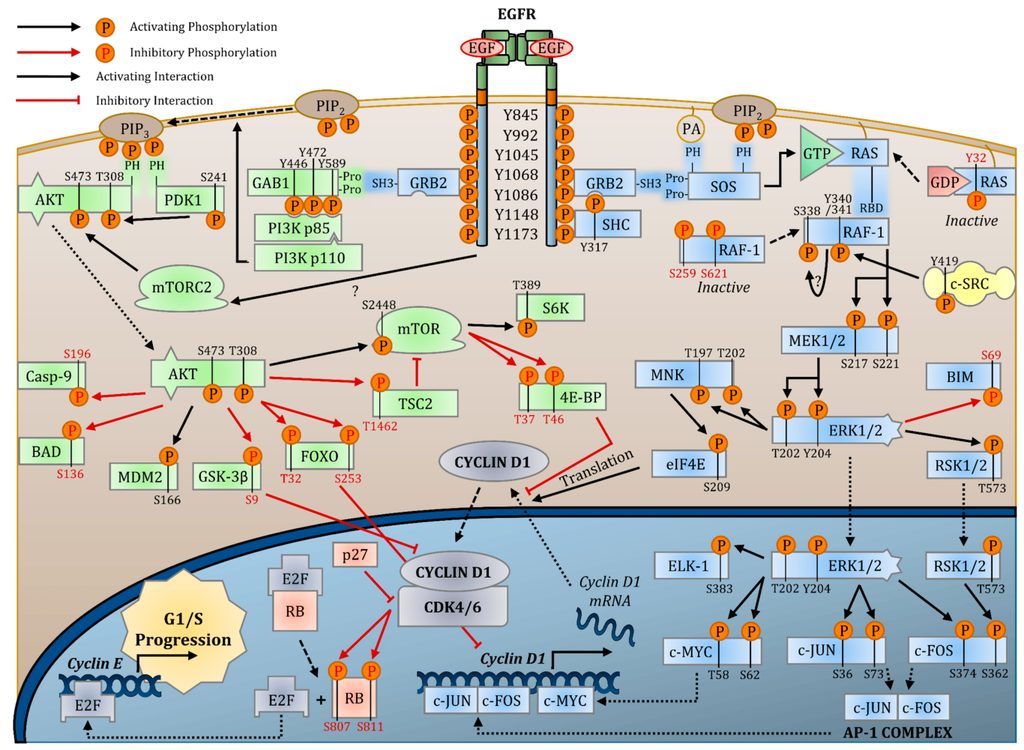
The EGFR (Epidermal Growth Factor Receptor) protein signaling pathway is a critical cellular pathway that regulates various cellular processes, including cell growth, differentiation, proliferation, and survival. EGFR is a receptor tyrosine kinase that is activated upon binding to specific ligands, such as epidermal growth factor (EGF) and transforming growth factor alpha (TGF-α).
When ligands bind to EGFR, it induces receptor dimerization, leading to autophosphorylation of specific tyrosine residues within the receptor's intracellular domain. This autophosphorylation serves as a molecular switch, activating downstream signaling cascades. The activated EGFR can then recruit and phosphorylate various intracellular signaling molecules, including Ras, Raf, MEK, and ERK, initiating the MAPK/ERK pathway. Activation of this pathway results in the regulation of gene expression, promoting cell growth and proliferation.
In addition to the MAPK/ERK pathway, EGFR signaling also activates the PI3K/AKT/mTOR pathway. Phosphorylation of AKT leads to the activation of mTOR, which is involved in regulating protein synthesis and cell survival.
Furthermore, EGFR signaling can also trigger the JAK/STAT pathway, which regulates cell differentiation and immune responses.
The EGFR pathway's activation is tightly regulated to prevent uncontrolled cell growth and proliferation. Aberrant EGFR signaling is associated with various diseases, including cancer. Overexpression or mutations of EGFR can lead to constitutive activation of the pathway, promoting tumor growth and progression.
EGFR and Its Impact on Tumors
Abnormal expression of EGFR (Epidermal Growth Factor Receptor) is closely associated with tumor development, growth, and metastasis in various cancer types. Overexpression of EGFR leads to continuous stimulation of tumor cells by proliferation and survival signals, disrupting the normal regulation of cell cycle and apoptosis. As a result, uncontrolled tumor growth is promoted.
Notably, mutations in EGFR are particularly prevalent in tumors such as non-small cell lung cancer and colorectal cancer. These mutations cause abnormal activation of EGFR even in the absence of external ligands, leading to resistance to standard anticancer therapies.
Given the pivotal role of EGFR in tumor cells, it has become a prime target for cancer therapy research. Targeted therapeutic drugs designed to inhibit EGFR have been developed and widely employed, including tyrosine kinase inhibitors (TKIs) and monoclonal antibodies. By interfering with EGFR activation, these drugs effectively hinder tumor cell proliferation and growth, offering a promising approach to cancer inhibition.
The Significance of EGFR Protein Research
The research on EGFR protein holds great importance and offers valuable insights into various aspects:
- Key Cell Signal Transduction Pathway: EGFR (Epidermal Growth Factor Receptor) serves as a receptor-type tyrosine kinase involved in both extracellular and intracellular signal transmission. The EGFR signaling pathway significantly regulates crucial biological processes like cell growth, proliferation, differentiation, migration, and apoptosis.
- Implications in Cancer Occurrence and Development: Abnormalities in EGFR, such as overexpression, mutation, or activation, are closely linked to the development and progression of various cancers. Dysregulated EGFR activation can lead to uncontrolled cell growth, driving tumor formation and metastasis. Thus, studying EGFR's role in cancer helps uncover tumorigenesis mechanisms and potential therapeutic targets.
- Individualized Treatment Strategies: EGFR's pivotal role in numerous cancers has led to the development of EGFR pathway inhibitors as vital treatment options. By analyzing EGFR expression and mutations in tumors, personalized treatment plans can be tailored for patients, ensuring the selection of the most suitable drugs and treatment approaches, thereby enhancing treatment effectiveness and reducing side effects.
- Drug Development Advancements: EGFR pathway inhibitors, including EGFR inhibitors and anti-EGFR monoclonal antibodies, have become mainstays in cancer treatment. Comprehensive research on EGFR protein's structure and function guides the development and design of novel drugs, improving drug selectivity and efficacy.
Clinical Significance of EGFR
The clinical significance of EGFR (Epidermal Growth Factor Receptor) protein is vast and holds utmost importance in various medical domains:
- Cancer Diagnosis and Prognosis: EGFR is a critical biomarker for several cancer types, including lung, breast, colorectal, and head and neck cancers. Its overexpression or mutations in cancer cells often indicate a more aggressive disease with a poorer prognosis. Detecting EGFR status in cancer patients helps in selecting appropriate treatment strategies and predicting patient outcomes.
- Targeted Cancer Therapy: EGFR has become a major target in cancer treatment. Drugs that specifically inhibit EGFR, such as EGFR tyrosine kinase inhibitors (TKIs) and monoclonal antibodies, have been successfully developed and used to treat certain cancer types. Targeted therapy against EGFR blocks signals promoting cancer cell growth and division, leading to tumor regression and improved survival rates.
- Personalized Medicine: EGFR status often guides personalized treatment decisions. Patients with EGFR-positive tumors may benefit from targeted therapy, while those with EGFR-negative tumors might require alternative treatment options. This personalized approach ensures patients receive the most effective and tailored treatment for their specific cancer.
- Drug Resistance: Despite initial effectiveness, EGFR-targeted therapies can face drug resistance over time. Studying EGFR mutations and resistance mechanisms is essential to develop new treatment strategies that overcome drug resistance and prolong the benefits of targeted therapy.
- Potential Biomarker for Other Diseases: Beyond cancer, EGFR has been implicated in cardiovascular diseases, neurodegenerative disorders, and inflammatory conditions. Research into EGFR's role in these diseases may lead to the development of new therapeutic approaches.
- Companion Diagnostics: EGFR testing has become a companion diagnostic tool for certain targeted therapies. Identifying EGFR mutations or overexpression helps identify patients likely to respond to specific treatments and avoids exposing non-responsive patients to potentially ineffective therapies.
In conclusion, the clinical significance of EGFR protein lies in its role as a biomarker for cancer diagnosis, prognosis, and targeted therapy. Understanding EGFR biology and its clinical implications has paved the way for personalized medicine and improved treatment outcomes for cancer patients. Ongoing research on EGFR continues to shed light on its involvement in various diseases and may uncover new therapeutic opportunities in the future.
EGFR Protein
Recombinant Human Epidermal growth factor receptor(EGFR),partial (Active)
High Purity Validated by SDS-PAGE
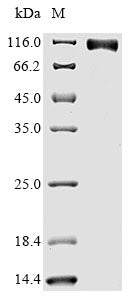
(Tris-Glycine gel) Discontinuous SDS-PAGE (reduced) with 5% enrichment gel and 15% separation gel.
Excellent Bioactivity Validated by Functional ELISA
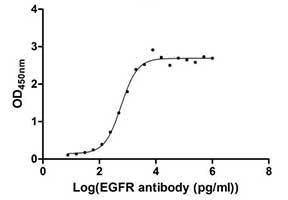
Measured by its binding ability in a functional ELISA. Immobilized EGFR at 1 μg/ml can bind human EGFR antibody, the EC50 of human EGFR protein is 2.867-3.571 ng/ml. Click here for more EGFR Synonym:ERBB, mENA, ERBB1, HER1
References:
[1] Lo, HW., Hung, MC. Nuclear EGFR signalling network in cancers: linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br J Cancer 94, 184–188 (2006). https://doi.org/10.1038/sj.bjc.6602941
[2] Guy PM, Platko JV, Cantley LC, Cerione RA, Carraway KL., III Insect cell-expressed p180(erbB3) possesses an impaired tyrosine kinase activity. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(17):8132–8136.
[3] Plowman GD, Whitney GS, Neubauer MG, et al. Molecular cloning and expression of an additional epidermal growth factor receptor-related gene. Proceedings of the National Academy of Sciences of the United States of America. 1990;87(13):4905–4909.
[4] Sierke SL, Cheng K, Kim H-H, Koland JG. Biochemical characterization of the protein tyrosine kinase homology domain of the ErbB3 (HER3) receptor protein. Biochemical Journal. 1997;322(3):757–763.
[5] Citri A, Skaria KB, Yarden Y. The deaf and the dumb: the biology of ErbB-2 and ErbB-3. Experimental Cell Research. 2003;284(1):54–65.
[6] Morandell S, Stasyk T, Skvortsov S, Ascher S, Huber LA. Quantitative proteomics and phosphoproteomics reveal novel insights into complexity and dynamics of the EGFR signaling network. Proteomics. 2008;8(21):4383–4401.
[7] Siwak DR, Carey M, Hennessy BT, et al. Targeting the epidermal growth factor receptor in epithelial ovarian cancer: current knowledge and future challenges. J Oncol. 2010;2010:568938. doi:10.1155/2010/568938
[8] Adams TE, McKern NM, Ward CW. Signalling by the type 1 insulin-like growth factor receptor: interplay with the epidermal growth factor receptor. Growth Factors. 2004;22(2):89–95.
[9] Jones HE, Gee JMW, Hutcheson IR, Knowlden JM, Barrow D, Nicholson RI. Growth factor receptor interplay and resistance in cancer. Endocrine-Related Cancer. 2006;13(supplement 1):S45–S51.
[10] Qiu L, Zhou C, Sun Y, et al. Crosstalk between EGFR and TrkB enhances ovarian cancer cell migration and proliferation. International Journal of Oncology. 2006;29(4):1003–1011.
[11] Gschwind A, Zwick E, Prenzel N, Leserer M, Ullrich A. Cell communication networks: epidermal growth factor receptor transactivation as the paradigm for interreceptor signal transmission. Oncogene. 2001;20(13):1594–1600.
[12] Shida D, Kitayama J, Mori K, Watanabe T, Nagawa H. Transactivation of epidermal growth factor receptor is involved in leptin-induced activation of Janus-activated kinase 2 and extracellular signal-regulated kinase 1/2 in human gastric cancer cells. Cancer Research. 2005;65(20):9159–9163.
[13] Wee P, Wang Z. Epidermal Growth Factor Receptor Cell Proliferation Signaling Pathways. Cancers (Basel). 2017;9(5):52. Published 2017 May 17. doi:10.3390/cancers9050052




