Deciphering the TCR/CD3 Complex: A Gateway to Immune Hallmarks
What is CD3
CD3, also known as Cluster of Differentiation 3, is a pivotal protein molecule situated on the cell membrane, serving as a vital marker within the immune system. Present on the cell membrane of T lymphocytes, CD3 is an integral component of the T cell receptor (TCR) complex, playing a pivotal role in both signal transmission and the identification of foreign antigens.
Comprising a diverse set of protein chains—gamma, delta, epsilon, and zeta—the CD3 complex forms through non-covalent interactions, resulting in a sophisticated molecular structure that tightly associates with the TCR. Upon TCR binding with an antigen, the intracellular signaling module within the CD3 chain becomes activated, initiating a sequence of signal transduction cascades. These cascades culminate in the activation, proliferation, and execution of T cell functions, such as cytokine secretion and target cell elimination.
CD3's significance extends to immune surveillance, the establishment of immune memory, and the recognition of antigens with specificity. Its expression profile and functional attributes may vary across distinct T cell categories, including CD4+ helper T cells and CD8+ cytotoxic T cells.
Given its pivotal role in immune responses, CD3 holds therapeutic implications for numerous immune-related disorders. For instance, in scenarios like organ transplantation and autoimmune diseases, modulation of T cell immune responses can be achieved by targeting CD3 signaling. This modulation aids in mitigating tissue rejection and restraining excessive immune reactions.
In conclusion, CD3's intricate involvement in immune responses highlights its importance. Through its interactions with TCR and its role in signal transduction, CD3 ensures the sensitive recognition of antigens and the regulation of immune functions. Its intricate role contributes to maintaining immune equilibrium and fostering a robust defense against infections.
The Role of TCR
T cell receptor (TCR), situated on the surface of T lymphocytes, stands as a pivotal player within the immune system. Its primary role lies in orchestrating T cell responses against an array of foreign antigens and aberrant cells through antigen recognition.
In the event of foreign invaders, such as bacteria or viruses, antigen-presenting cells diligently fragment these intruders and present the fragments on cell surface MHC molecules. Upon T cell encounter with these MHC-antigen combinations, TCR binds, thereby forming TCR-MHC-antigen complexes. The specificity of this binding empowers T cells to detect antigens, thus precipitating an immune reaction.
Following antigen binding, a cascade of signaling events initiates within the T cell. Partnering with TCR in this signaling endeavor is CD3, a protein of paramount importance. These signals instigate the activation and phosphorylation of a sequence of molecules, culminating in T cell activation. The activated T cells then differentiate into distinctive effector cells: CD8+ cytotoxic T cells, capable of eliminating infected cells, and CD4+ helper T cells, pivotal for secreting cytokines that aid fellow immune cells.
Furthermore, the role of TCR transcends into immune memory. Once T cells are triggered, they undergo differentiation into memory T cells, preserving their capacity to recognize specific antigens over extended periods. This facilitates a swifter and more robust immune retort upon subsequent encounters with the same antigen, effectively bolstering the immune response.
In conclusion, TCR's pivotal role in immune responses becomes evident. Its duality in antigen recognition and subsequent signaling, combined with its pivotal role in immune memory, underscores its importance in maintaining an effective immune system ready to combat potential threats.
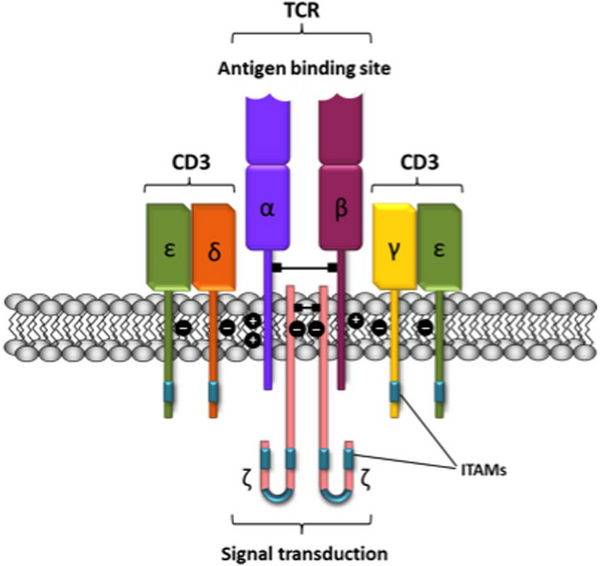
Unraveling the Structure of the TCR/CD3 Complex
T cell receptors can be categorized into two subgroups: TCRαβ and TCRγδ, based on the type of heterodimeric receptors they form. The αβ and γδ chains within these subgroups harbor polymorphic subunits that exhibit striking similarities in immunoglobulin variable regions (V) and constant regions (C). The primary role of the V region is to recognize and bind antigen polypeptides. However, the intracellular region (cytoplasmic tail) of the TCR is notably brief, lacking the capacity to initiate signaling upon binding to peptide major histocompatibility complex (pMHC) class I or class II molecules.
To activate the essential downstream pathways required for immune cell activation, the α/β chain of the TCR must establish a robust TCR/CD3 complex with 8 peptide chains through salt bridges. This intricate binding also involves the γ/ε chain heterodimeric form of the signaling molecule complex CD3 protein. This elaborate structure enables the transmission of stimulatory signals from T cells into the cell, initiating an intracellular cascade reaction.
Remarkably, the TCR/CD3 complex houses a total of 10 ITAMs, rendering it exceedingly sensitive to target antigens. Drugs that specifically target CD3 can either stimulate or inhibit T cell activation signal transduction. These drugs work by recognizing CD3ε chains, which naturally form heterodimers. Through the coordinated action of cytokines like IL2 and IL1a, these drugs can facilitate non-MHC-restricted tumor cell killing. Furthermore, they offer potential in treating organ transplant rejections and autoimmune diseases.
In conclusion, understanding the complex interplay within the TCR/CD3 complex sheds light on its intricate role in orchestrating immune responses. By manipulating these interactions, we can explore novel avenues for therapeutic interventions in diverse medical scenarios.
Decoding the TCR/CD3 Signaling Pathway
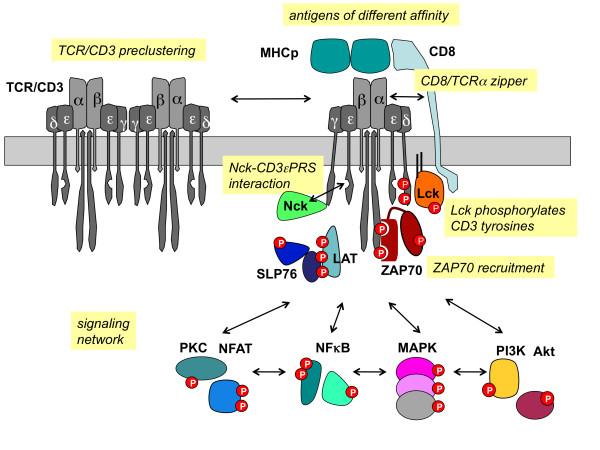
The TCR/CD3 signaling pathway is pivotal to T cell immune responses, representing their core mechanism. When the T cell receptor (TCR) engages with an antigen, it interfaces with the CD3 complex. This interaction sets in motion internal signaling pathways, instigating a sequence of molecular events that culminate in the activation and functional engagement of T cells.
Signaling is instigated within the intracellular domain of the TCR/CD3 complex, encompassing multiple intracellular tyrosine residues in the CD3 chain, which undergo phosphorylation upon complex activation. These phosphorylation events activate tyrosine kinases, including Lck. Subsequently, these kinases further phosphorylate other CD3 chains, initiating a cascade that amplifies the signaling process.
Following a series of molecular exchanges, the activated CD3 chain proceeds to phosphorylate another CD3 chain known as the zeta chain. This event leads to the binding and activation of ZAP-70, a crucial protein kinase. ZAP-70's activation serves as a pivotal juncture, setting off other signaling proteins such as LAT (linking activated T cells), SOS (triggering the Ras pathway), and PLCγ1 (stimulating the calcium channel pathway). This intricate interplay generates a cascade of molecular signals, ultimately inducing heightened intracellular calcium concentrations, elevated cytokine gene expression, and T cell activation.
This signaling cascade initiates the differentiation and proliferation of T cells, prompting them to release cytokines that coordinate and regulate immune responses. Furthermore, this activation primes T cells for their cytotoxic functions, enabling them to eliminate infected or aberrant cells.
In summary, the TCR/CD3 signaling pathway stands as a pivotal regulatory mechanism for T cell immune responses. Its central role in sustaining immune balance, bolstering the body's defense against infections, and orchestrating immune reactions underscores its significance in maintaining overall health.
Unveiling the Clinical Importance of CD3 Detection
The clinical detection of CD3 holds paramount significance across various domains, encompassing the following pivotal aspects:
- Immune Function Assessment: The quantification of CD3 expression serves as a tool to assess the immune functionality in patients. Post immunodeficiency disorders or immunosuppressive treatments, T cell counts and activities might decline, leading to diminished CD3 expression. By scrutinizing CD3 expression, physicians can gauge the patient's immune status, steer treatment strategies, and monitor the trajectory of the ailment.
- Transplant Rejection Surveillance: In the realm of organ transplantation, CD3 detection assumes a pivotal role in graft rejection surveillance. During rejection episodes, the recipient's immune system might mount an attack against the transplanted organ, resulting in an escalation of CD3+ T cells. Routine monitoring of CD3 counts empowers medical professionals to swiftly identify and manage rejection incidents.
- Immune Disorder Diagnosis: Certain immune disorders, such as autoimmune conditions and allergies, often culminate in anomalous T cell activation or aberrant CD3 expression. A thorough examination of CD3 expression can aid physicians in ascertaining the diagnosis and classifying these disorders more accurately.
- Therapeutic Efficacy Monitoring: In the realm of immune disorder treatments, such as employing T cell inhibitors for autoimmune ailments or resorting to immunosuppressive therapies post-organ transplantation, CD3 monitoring assumes a pivotal role in evaluating treatment efficacy. Successful treatment outcomes are frequently accompanied by a reduction in CD3+ T cells.
In conclusion, the clinical assessment of CD3 carries profound implications, spanning a gamut of facets including immune function evaluation, transplant rejection vigilance, immune disorder diagnostics, and treatment efficacy scrutiny. This comprehensive approach to CD3 detection serves as a cornerstone in optimizing patient care and contributing to the realm of clinical immunology.
CD3 Protein
References:
[1] Kolb HJ, Mittermüller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76(12):2462-2465.
[2] Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555-562.
[3] Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319(25):1676-1680. doi:10.1056/NEJM198812223192527
[4] Dudley ME, Wunderlich JR, Robbins PF, et al. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298(5594):850-854. doi:10.1126/science.1076514
[5] Rosenberg SA, Dudley ME. Cancer regression in patients with metastatic melanoma after the transfer of autologous antitumor lymphocytes. Proc Natl Acad Sci U S A. 2004;101 Suppl 2(Suppl 2):14639-14645. doi:10.1073/pnas.0405730101
[6] Rooney CM, Smith CA, Ng CY, et al. Use of gene-modified virus-specific T lymphocytes to control Epstein-Barr-virus-related lymphoproliferation. Lancet. 1995;345(8941):9-13. doi:10.1016/s0140-6736(95)91150-2
[7] Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549-1555.
[8] Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549-1555.
[9] Haque T, Taylor C, Wilkie GM, et al. Complete regression of posttransplant lymphoproliferative disease using partially HLA-matched Epstein Barr virus-specific cytotoxic T cells. Transplantation. 2001;72(8):1399-1402. doi:10.1097/00007890-200110270-00012
[10] Franco R, Martínez-Pinilla E, Lanciego JL, Navarro G. Basic Pharmacological and Structural Evidence for Class A G-Protein-Coupled Receptor Heteromerization. Front Pharmacol. 2016;7:76. Published 2016 Mar 31. doi:10.3389/fphar.2016.00076
[11] Borroto-Escuela DO, Van Craenenbroeck K, Romero-Fernandez W, et al. Dopamine D2 and D4 receptor heteromerization and its allosteric receptor-receptor interactions. Biochem Biophys Res Commun. 2011;404(4):928-934. doi:10.1016/j.bbrc.2010.12.083
[12] Daniels DJ, Kulkarni A, Xie Z, Bhushan RG, Portoghese PS. A bivalent ligand (KDAN-18) containing delta-antagonist and kappa-agonist pharmacophores bridges delta2 and kappa1 opioid receptor phenotypes. J Med Chem. 2005;48(6):1713-1716. doi:10.1021/jm034234f
[13] Edwards PC, Li J, Burghammer M, et al. Crystals of native and modified bovine rhodopsins and their heavy atom derivatives. J Mol Biol. 2004;343(5):1439-1450. doi:10.1016/j.jmb.2004.08.089
[14] Fotiadis D, Jastrzebska B, Philippsen A, Müller DJ, Palczewski K, Engel A. Structure of the rhodopsin dimer: a working model for G-protein-coupled receptors. Curr Opin Struct Biol. 2006;16(2):252-259. doi:10.1016/j.sbi.2006.03.013
[15] Louis-Dit-Sully C, Kubatzky KF, Lindquist JA, Blattner C, Janssen O, Schamel WW. Meeting report: Signal transduction meets systems biology. Cell Commun Signal. 2012;10(1):11. Published 2012 Apr 30. doi:10.1186/1478-811X-10-11
[16] Ginés S, Hillion J, Torvinen M, et al. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc Natl Acad Sci U S A. 2000;97(15):8606-8611. doi:10.1073/pnas.150241097
[17] Hiller C, Kühhorn J, Gmeiner P. Class A G-protein-coupled receptor (GPCR) dimers and bivalent ligands. J Med Chem. 2013;56(17):6542-6559. doi:10.1021/jm4004335




