Balancing the Immune See-Saw: CTLA-4 and CD28 in Immune Regulation
Unveiling CTLA-4: A Crucial Immune Regulator
CTLA-4, short for Cytotoxic T-Lymphocyte Antigen 4, stands as a pivotal immunoregulatory protein within the immunoglobulin superfamily. This protein assumes a critical role in orchestrating activity and equilibrium within the immune system. Predominantly, CTLA-4 governs the immune response by curbing the activity of T cells, ensuring a restrained immune reaction towards the body's own tissues.
The Structure of CTLA-4
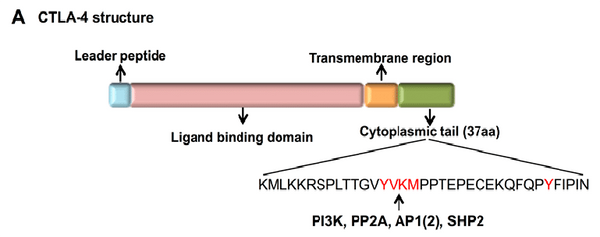
The CTLA-4 structure is composed of three vital components: the ligand binding region, transmembrane region, and cytoplasmic tail, each orchestrating a pivotal role in the regulation of T cell activity.
- Ligand Binding Region: Nested within CTLA-4's extracellular domain, the ligand binding region comprises a Variable IgV (immunoglobulin variable) domain and a Constant IgC (immunoglobulin constant) domain. This section interacts with its primary ligands, known as the B7 molecules. When CTLA-4 binds to B7, it outcompetes CD28, another T cell receptor, for the same binding site on T cells. This competitive binding effectively suppresses T cell activation.
- Transmembrane Region: Linking the extracellular domain with the cytoplasmic tail, the transmembrane region spans the cell membrane, anchoring CTLA-4 within the T cell membrane. Its responsibility extends to maintaining the stable placement of CTLA-4 and facilitating interactions with other cell components.
- Cytoplasmic Tail: Situated within the T cell, on the cytoplasmic side of the cell membrane, the cytoplasmic tail plays a role in intracellular signaling processes. Following CTLA-4's binding to B7, its cytoplasmic tail transmits an inhibitory signal, which dampens T cell activation and proliferation. This mechanism is essential in fine-tuning the immune response's intensity, preventing excessive immune reactions.
In essence, the ligand binding region, transmembrane region, and cytoplasmic tail of CTLA-4 jointly regulate T cell immune activity and uphold immune equilibrium. This regulatory machinery is fundamental for the immune system's proper function and holds implications in fields like cancer immunotherapy and immune-related disorders.
Molecular and Cell Biology of the CTLA-4 Pathway
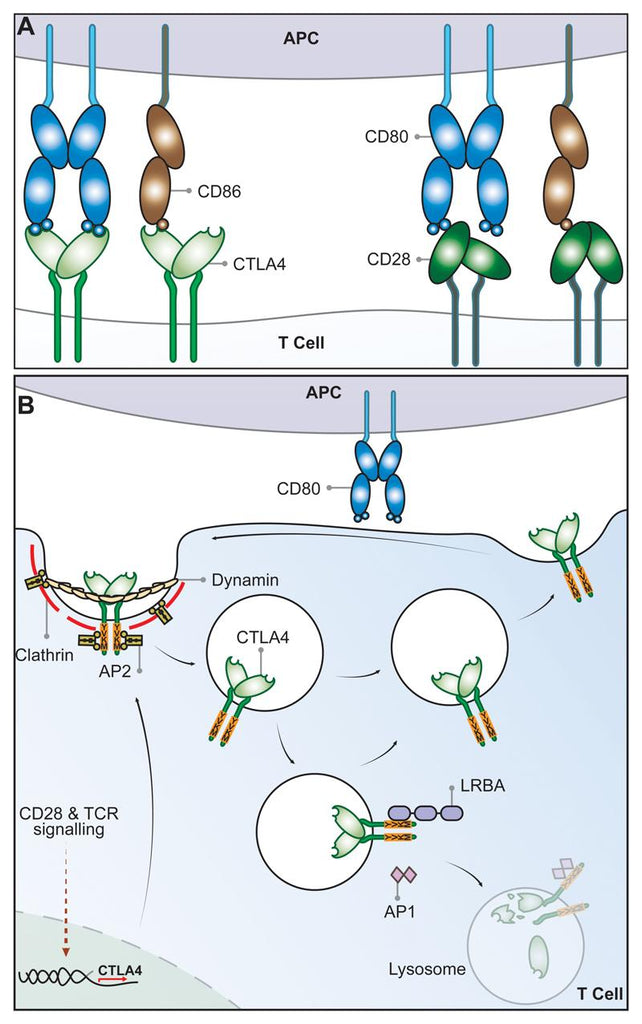
CTLA-4 and CD28 Share Two Ligands, CD80 and CD86
Cytotoxic T Lymphocyte antigen 4 (CTLA-4) (CD152) and CD28, homologous receptors expressed by both CD4+ and CD8+ T cells, wield opposing roles in T cell activation. Both receptors share a pair of ligands found on the surface of antigen presenting cells (APCs). CD28 engages the CD80 dimer with relatively high affinity and the CD86 monomer with lower affinity, instigating T cell co-stimulation alongside T cell receptor (TCR) signals. Conversely, interactions between these ligands and CTLA-4 lead to T cell suppression, although the exact mechanisms remain incompletely elucidated. CTLA-4 binds to both ligands with greater affinity and avidity than CD28[2-4], forming the most avid interaction with CTLA-4-CD80 and the weakest with CD28-CD86. This suggests that CTLA-4 might compete with CD28 for ligand binding, acting as an antagonist of CD28-mediated co-stimulation[5-6]. These interactions are presumed to occur at the immune synapse between T cells and APCs, where CTLA-4 recruits CD80, thereby curbing its interactions with CD28[7-8].
Despite well-defined biophysical traits of CD80 and CD86, their functional disparities remain less evident. These ligands are often collectively termed B7 molecules or CD80/CD86. Found on APCs such as dendritic cells and B cells, they exhibit distinct expression patterns, inducibility, and kinetics[9-11]. Knockout mice display impaired immune responses consistent with diminished CD28 co-stimulation, with CD86 deficiency possibly resulting in a more severe phenotype[10]. There exists substantial variation in ligand expression, complicating generalizations. CD86 is often constitutively expressed on dendritic cells (DCs), escalating post-inflammatory stimuli. In contrast, DCs are believed to upregulate CD80 later. Human peripheral blood monocytes typically express CD86 and not CD80, while certain human T cells might express both CD80 and CD86 under different activation contexts[12-13]. Consequently, the current differences between these ligands largely revolve around diverse expression patterns, with definitive functional distinctions yet to materialize.
Cell Biology of CTLA-4
While CD28 maintains a constant presence on the plasma membrane to co-stimulate T cells, CTLA-4 predominantly resides within intracellular vesicles in FoxP3+ Treg cells or activated conventional T cells. This cellular localization arises from CTLA-4's ongoing endocytosis from the plasma membrane, resulting in about 90% of CTLA-4 being sequestered intracellularly[15-16]. The process of CTLA-4 endocytosis is astonishingly swift, with over 80% of surface CTLA-4 being internalized within just five minutes[17]. Once internalized, CTLA-4 molecules seem to be either recycled back to the plasma membrane or degraded within lysosomal compartments. However, the intricate mechanisms and functional implications of this process remain incompletely understood.
The post-endocytic fate and control of CTLA-4 trafficking is still poorly characterized, although experiments show that CTLA-4 lacking its 36-amino acid cytoplasmic tail is predominantly located at the cell surface[18]. A number of studies have therefore focused on identifying partners that interact with the cytoplasmic tail of CTLA-4 and on defining the role of its cytoplasmic domain. CTLA-4 endocytosis is dependent on clathrin due to its interaction with the µ2 subunit of the clathrin adaptor protein complex AP2[19] and is also dependent upon dynamin. AP2 targets the cytoplasmic tyrosine containing YVKM motif of CTLA-4 and can be disengaged when this motif is tyrosine-phosphorylated upon T cell activation[20]. However, the immunological settings where the CTLA-4–AP-2 interaction is disrupted are unclear since activated T cells and Treg continue to endocytose CTLA-4. The cytoplasmic domain sequence and trafficking behavior of CTLA-4 are highly conserved in mammals whereas some animals such as fish lack this endocytic motif,[21-22] resulting in predominantly surface expression. This suggests that endocytosis may be a later adaptation of CTLA-4. Overall, CD28 and CTLA-4 differ profoundly in sub-cellular location despite binding to the same ligands and, given the conservation of these features, it seems likely they are pivotal to the functioning of the system.
The significance of CTLA-4 trafficking was underscored in patients with Lipopolysaccharide-responsive and beige-like anchor protein (LRBA) deficiency[23]. This BEACH domain-containing protein[24] seems to regulate CTLA-4 turnover and primarily co-localizes with recycling (Rab11+) endosomes. LRBA deficiency leads to heightened CTLA-4 degradation, contributing to autoimmunity, implying that LRBA might hinder CTLA-4 trafficking to lysosomes and encourage recycling. This is possibly achieved through LRBA binding to CTLA-4's YVKM consensus sequence. Further control of CTLA-4 trafficking and cellular distribution might be orchestrated by the TRIM/LAX/Rab8 complex, involved in post-Golgi CTLA-4 transport to the cell surface[25]. Recent reports have also revealed CTLA-4 interactions involving PKC-eta and the PIX-PAK pathway, potentially influencing Treg-APC interactions. Deficient PKC-eta in Tregs is associated with impaired CD86 depletion from APCs by Tregs and heightened Treg motility[26].
CTLA-4/CD28 Signaling Pathway
CD28 and CTLA-4 engage with an array of intracellular signaling proteins, orchestrating a complex network of cellular responses. CD28 couples with PI3K and Grb2 through SH2 domain binding to the Tyr–Val–Asn–Met (YMNM) motif. PI3K generates PI3,4P2 and PI3,4,5P3 lipids, while Grb2 interacts with Sos1, an activator of the GTPase p21ras. CD28–Grb2 association is pivotal for Vav1 phosphorylation and activation. Consequently, Vav1 activates Rac1, which, in turn, triggers the serine/threonine kinase JNK. Vav1's influence also extends to Cdc42 activation in WASP engagement and the membrane anchoring of PKCθ. Protein tyrosine phosphatase 1 (SHP-1) interacts with Vav1, Grb2, and Sos1, mitigating CD28's signals. The CD28–PYAPP motif, positioned towards the C-terminus, binds to FLNA and colocalizes with PKCθ and CD28. Mutations in this motif disrupt PKCθ formation within a cSMAC. CD28 endocytosis is modulated by a trio of mediators: PI3K, WASP, and SNX9. Mutations impairing PI3K binding disrupt internalization through a clathrin-dependent mechanism. SNX9's SH3 domain binds WASP, while its PX domain interacts with PI3K's p85 subunit and its product PIP3. Clathrin-containing endocytic vesicles host the colocalization of WASP, SNX9, PI3K, and CD28 after TCR/CD28 costimulation.
On the other hand, CTLA-4 binds to PI3K using its YVKM motif as well as phosphatases SHP-2 and PP2A. Phosphatases have been postulated to generate negative signals. CTLA-4 can also block the expression of lipid rafts (GEMs) and the induction by the TCR of ZAP-70 microcluster formation. CTLA-4, cytotoxic T-lymphocyte antigen-4; PI3K, phosphatidylinositol 3-kinase; SH2, src homology 2; WASP, Wiskott–Aldrich syndrome protein; PKCθ, protein kinase C θ; cSMAC, central supramolecular activation cluster; SNX, sorting nexin; TCR, T-cell receptor; YVKM, Val–Tyr–Val–Lys–Met; SHP-1, SH2-domain-containing protein tyrosine phosphatase 1; GEM, glycolipid-enriched microdomain.
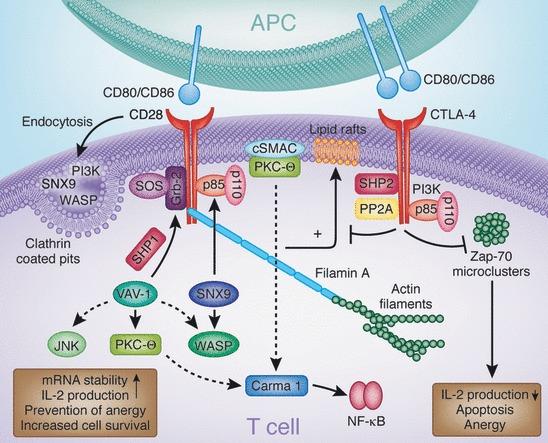
Fig.3 Signaling molecules involved in CD28 and CTLA-4 function [27]
CTLA-4/CD28 Protein
Recombinant Human CTLA4 /CTLA-4 Protein
Synonym:ALPS5 CD CD 152 CD152 CD152 antigen CD152 isoform Celiac disease 3 CELIAC3 CTLA 4 CTLA-4 CTLA4 CTLA4_HUMAN Cytotoxic T cell associated 4 Cytotoxic T lymphocyte antigen 4 Cytotoxic T lymphocyte associated 4 Cytotoxic T lymphocyte associated 4, soluble isoform, included Cytotoxic T lymphocyte associated antigen 4 Cytotoxic T lymphocyte associated antigen 4 short spliced form Cytotoxic T lymphocyte associated protein 4 Cytotoxic T lymphocyte associated serine esterase 4 Cytotoxic T lymphocyte protein 4 Cytotoxic T-lymphocyte protein 4 Cytotoxic T-lymphocyte-associated antigen 4 GRD4 GSE ICOS IDDM12 insulin-dependent diabetes mellitus 12 Ligand and transmembrane spliced cytotoxic T lymphocyte associated antigen 4 OTTHUMP00000216623
Recombinant Human T-Cell-Specific Surface Glycoprotein Cd28 (CD28) Protein (hFc)
Synonym:CD 28 CD28 CD28 antigen CD28 molecule CD28_HUMAN MGC138290 T cell antigen CD28 T cell specific surface glycoprotein T cell specific surface glycoprotein CD28 T-cell-specific surface glycoprotein CD28 TP44
References:
[1] Zhao Y, Yang W, Huang Y, Cui R, Li X, Li B. Evolving Roles for Targeting CTLA-4 in Cancer Immunotherapy. Cell Physiol Biochem. 2018;47(2):721-734. doi: 10.1159/000490025. Epub 2018 May 22. PMID: 29794465.
[2] Schwartz JC, Zhang X, Fedorov AA, Nathenson SG, Almo SC. Structural basis for co-stimulation by the human CTLA-4/B7-2 complex. Nature. 2001 Mar 29;410(6828):604-8. doi: 10.1038/35069112. PMID: 11279501.
[3] Stamper CC, Zhang Y, Tobin JF, Erbe DV, Ikemizu S, Davis SJ, Stahl ML, Seehra J, Somers WS, Mosyak L. Crystal structure of the B7-1/CTLA-4 complex that inhibits human immune responses. Nature. 2001 Mar 29;410(6828):608-11. doi: 10.1038/35069118. Erratum in: Nature 2001 May 31;411(6837):617. PMID: 11279502.
[4] Collins AV, Brodie DW, Gilbert RJ, Iaboni A, Manso-Sancho R, Walse B, Stuart DI, van der Merwe PA, Davis SJ. The interaction properties of costimulatory molecules revisited. Immunity. 2002 Aug;17(2):201-10. doi: 10.1016/s1074-7613(02)00362-x. PMID: 12196291.
[5] Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997 Oct;7(4):445-50. doi: 10.1016/s1074-7613(00)80366-0. PMID: 9354465.
[6] Walker LS, Sansom DM. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat Rev Immunol. 2011 Nov 25;11(12):852-63. doi: 10.1038/nri3108. PMID: 22116087.
[7] Pentcheva-Hoang T, Egen JG, Wojnoonski K, Allison JP. B7-1 and B7-2 selectively recruit CTLA-4 and CD28 to the immunological synapse. Immunity. 2004 Sep;21(3):401-13. doi: 10.1016/j.immuni.2004.06.017. PMID: 15357951.
[8] Yokosuka T, Kobayashi W, Takamatsu M, Sakata-Sogawa K, Zeng H, Hashimoto-Tane A, Yagita H, Tokunaga M, Saito T. Spatiotemporal basis of CTLA-4 costimulatory molecule-mediated negative regulation of T cell activation. Immunity. 2010 Sep 24;33(3):326-39. doi: 10.1016/j.immuni.2010.09.006. PMID: 20870175.
[9] Schweitzer AN, Sharpe AH. Studies using antigen-presenting cells lacking expression of both B7-1 (CD80) and B7-2 (CD86) show distinct requirements for B7 molecules during priming versus restimulation of Th2 but not Th1 cytokine production. J Immunol. 1998 Sep 15;161(6):2762-71. PMID: 9743334.
[10] Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997 Mar;6(3):303-13. doi: 10.1016/s1074-7613(00)80333-7. PMID: 9075931.
[11] Su KY, Watanabe A, Yeh CH, Kelsoe G, Kuraoka M. Efficient Culture of Human Naive and Memory B Cells for Use as APCs. J Immunol. 2016 Nov 15;197(10):4163-4176. doi: 10.4049/jimmunol.1502193. Epub 2016 Oct 10. PMID: 27815447; PMCID: PMC5111638.
[12] Sansom DM, Hall ND. B7/BB1, the ligand for CD28, is expressed on repeatedly activated human T cells in vitro. Eur J Immunol. 1993 Jan;23(1):295-8. doi: 10.1002/eji.1830230148. PMID: 7678229.
[13] Azuma M, Ito D, Yagita H, Okumura K, Phillips JH, Lanier LL, Somoza C. B70 antigen is a second ligand for CTLA-4 and CD28. Nature. 1993 Nov 4;366(6450):76-9. doi: 10.1038/366076a0. PMID: 7694153.
[14] Rowshanravan B, Halliday N, Sansom DM. CTLA-4: a moving target in immunotherapy. Blood. 2018 Jan 4;131(1):58-67. doi: 10.1182/blood-2017-06-741033. Epub 2017 Nov 8. PMID: 29118008; PMCID: PMC6317697.
[15] Linsley PS, Bradshaw J, Greene J, Peach R, Bennett KL, Mittler RS. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity. 1996 Jun;4(6):535-43. doi: 10.1016/s1074-7613(00)80480-x. PMID: 8673700.
[16] Shiratori T, Miyatake S, Ohno H, Nakaseko C, Isono K, Bonifacino JS, Saito T. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity. 1997 May;6(5):583-9. doi: 10.1016/s1074-7613(00)80346-5. PMID: 9175836.
[17] Qureshi OS, Kaur S, Hou TZ, Jeffery LE, Poulter NS, Briggs Z, Kenefeck R, Willox AK, Royle SJ, Rappoport JZ, Sansom DM. Constitutive clathrin-mediated endocytosis of CTLA-4 persists during T cell activation. J Biol Chem. 2012 Mar 16;287(12):9429-40. doi: 10.1074/jbc.M111.304329. Epub 2012 Jan 19. PMID: 22262842; PMCID: PMC3308817.
[18] Walker LS, Sansom DM. Confusing signals: recent progress in CTLA-4 biology. Trends Immunol. 2015 Feb;36(2):63-70. doi: 10.1016/j.it.2014.12.001. Epub 2015 Jan 9. PMID: 25582039; PMCID: PMC4323153.
[19] Chuang E, Alegre ML, Duckett CS, Noel PJ, Vander Heiden MG, Thompson CB. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J Immunol. 1997 Jul 1;159(1):144-51. PMID: 9200449.
[20] Zhang Y, Allison JP. Interaction of CTLA-4 with AP50, a clathrin-coated pit adaptor protein. Proc Natl Acad Sci U S A. 1997 Aug 19;94(17):9273-8. doi: 10.1073/pnas.94.17.9273. PMID: 9256472; PMCID: PMC23153.
[21] Bernard D, Riteau B, Hansen JD, Phillips RB, Michel F, Boudinot P, Benmansour A. Costimulatory receptors in a teleost fish: typical CD28, elusive CTLA4. J Immunol. 2006 Apr 1;176(7):4191-200. doi: 10.4049/jimmunol.176.7.4191. PMID: 16547256.
[22] Kaur S, Qureshi OS, Sansom DM. Comparison of the intracellular trafficking itinerary of ctla-4 orthologues. PLoS One. 2013;8(4):e60903. doi: 10.1371/journal.pone.0060903. Epub 2013 Apr 2. PMID: 23565286; PMCID: PMC3614947.
[23] Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, Zhang Y, Liu Z, Fritz JM, Marsh R, Husami A, Kissell D, Nortman S, Chaturvedi V, Haines H, Young LR, Mo J, Filipovich AH, Bleesing JJ, Mustillo P, Stephens M, Rueda CM, Chougnet CA, Hoebe K, McElwee J, Hughes JD, Karakoc-Aydiner E, Matthews HF, Price S, Su HC, Rao VK, Lenardo MJ, Jordan MB. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015 Jul 24;349(6246):436-40. doi: 10.1126/science.aaa1663. PMID: 26206937.
[24] Cullinane AR, Schäffer AA, Huizing M. The BEACH is hot: a LYST of emerging roles for BEACH-domain containing proteins in human disease. Traffic. 2013 Jul;14(7):749-66. doi: 10.1111/tra.12069. Epub 2013 Apr 24. PMID: 23521701; PMCID: PMC3761935.
[25] Banton MC, Inder KL, Valk E, Rudd CE, Schneider H. Rab8 binding to immune cell-specific adaptor LAX facilitates formation of trans-Golgi network-proximal CTLA-4 vesicles for surface expression. Mol Cell Biol. 2014 Apr;34(8):1486-99. doi: 10.1128/MCB.01331-13. Epub 2014 Feb 10. PMID: 24515439; PMCID: PMC3993577.
[26] Kong KF, Fu G, Zhang Y, Yokosuka T, Casas J, Canonigo-Balancio AJ, Becart S, Kim G, Yates JR 3rd, Kronenberg M, Saito T, Gascoigne NR, Altman A. Protein kinase C-η controls CTLA-4-mediated regulatory T cell function. Nat Immunol. 2014 May;15(5):465-72. doi: 10.1038/ni.2866. Epub 2014 Apr 6. PMID: 24705298; PMCID: PMC4040250.
[27] Rudd CE, Taylor A, Schneider H. CD28 and CTLA-4 coreceptor expression and signal transduction. Immunol Rev. 2009 May;229(1):12-26. doi: 10.1111/j.1600-065X.2009.00770.x. PMID: 19426212; PMCID: PMC4186963.





